Researchers in Germany have designed an organic reagent capable of inserting a single carbon atom into existing molecules – one of three group transfer reactions supported by the new compound. The designer reagent is easy to make and could become a powerful addition to the skeletal editing toolbox.
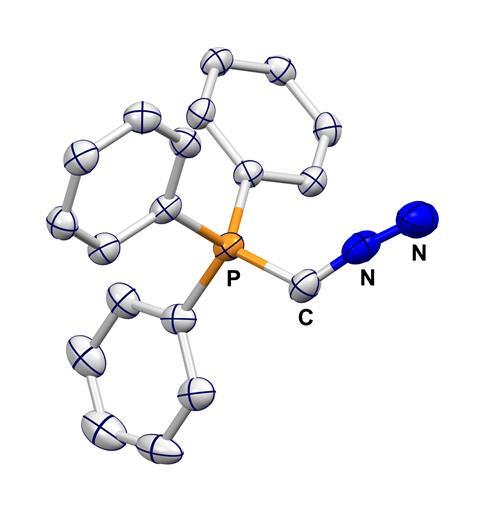
Carbon atom insertions are important transformations in organic chemistry and homologations like the Wittig reaction are staples of undergraduate chemistry. These processes typically use a dipolar reagent called an ylide which contains a (formally) negatively charged carbon atom attached to a (formally) positively charged heteroatom which is cleaved during the reaction. However, while this chemistry is well-established, many ylide reagents are synthesised using explosive azides and are unstable at room temperature, limiting both the practicality and scope of these techniques.
Aiming to expand the potential of carbon transfer reactions, Max Hansmann and his team at the Technical University of Dortmund in Germany set about designing an alternative ylide reagent. They envisaged a central carbon atom flanked by easily cleaved triphenylphosphine and diazo groups. ‘It’s very well known that you can cleave C–P and C–N bonds with this moiety so it would be the ideal reagent to transfer a carbon atom,’ he says.
With safety in mind, the team’s synthesis intentionally avoids problematic azide reagents, instead gently heating a diphosphorane precursor with nitrous oxide to prepare the proposed ylide. The resulting solid reagent displays high thermal stability and its cumulene-like structure – containing three consecutive double bonds – was confirmed by x-ray analysis.
The C–P and C–N bonds can be broken in subsequent reactions and the team began to explore how to direct the different possible group transfer processes. ‘You control the reactivity via the substrate you offer,’ Hansmann explains. ‘An α,β-unsaturated compound will typically react through a [3+2] cycloaddition, transferring CN2 to give a pyrazole. An electrophilic species like carbon monoxide undergoes an unusual substitution mechanism related to a transition metal ligand exchange, cleaving N2 and transferring CPPh3.’
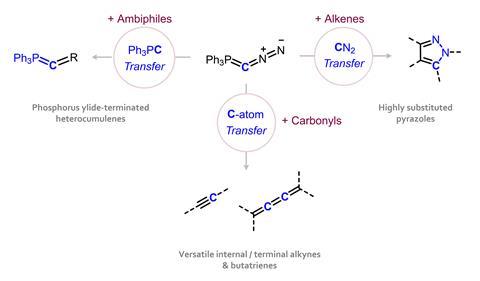
Most significantly, by cleaving both the triphenylphosphine and diazo groups, the reagent can transfer a single carbon atom to a carbonyl reagent, ultimately generating alkyne and butatriene products. ‘The first step is very similar to the Wittig reaction – a [2+2] cycloaddition with the carbonyl. Then you cleave off the triphenylphosphine to generate a diazoalkene, cleave off the N2 to get a vinylidene and then you have different pathways,’ he explains. ‘1-2 migration leads to the alkyne, or you dimerise and access the butatriene – it depends on the carbonyl.’ For Hansmann, this is the most valuable reactivity for the organic chemistry community – providing a new easy route to these important functional groups and overcoming the limitations of traditional ylide reagents.
Initially the team focused on establishing this carbon transfer reactivity for relatively simple substrates but they are now exploring its potential for larger and more complex systems. ‘There could be applications in skeletal editing, for example, enlarging rings and introducing a carbon atom at a later stage,’ Hansmann says. ‘It could also be interesting for the main group community as you can essentially play around with coordination complexes of carbon.’
For Mark Levin, an organic chemist at the University of Chicago in the US, the ylide’s potential as a tool in skeletal editing is the most exciting aspect of the work. ‘Reagent design is an underappreciated aspect of progress in organic chemistry and [in skeletal editing] there are a unique set of challenges which complicate the reagent design further,’ he says. ‘I think this reagent is going to be quite popular moving forward – it’s such a simple synthesis from the carbodiphosphorane and nitrous oxide, and the reactivity they have already shown is quite interesting.’
References
T Koike, J-K Yu and M M Hansmann, Science, 2024, DOI: 10.1126/science.ado4564













No comments yet