Enzymes encapsulated in a golden cage perform in extreme conditions
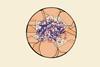
No room for the proteins to denature but reactants can still get through
Scientists in Israel have devised a new approach to enzyme immobilisation – encasing them in metallic gold. The caged enzymes are stable at extreme pHs and high temperatures, with one surviving at pH 13.