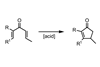
Nazarov cyclisation
We are all shaped by the opportunities afforded us, by the social structures and politics of our day
In 1906, Ivan Nazarov was born in the tiny village of Koshelevo, in the Russian province of Nizhni Novgoro to the east of Moscow. His family were peasants and Ivan worked daily as a farm labourer while attending the village school. After losing both parents before the age of fifteen, he and an elder brother assumed responsibility for their young siblings, and yet Ivan continued to study. Thoughtful and determined, here was a young man with prospects beyond his inherited life of hardship.