Google’s AI aces protein prediction competition
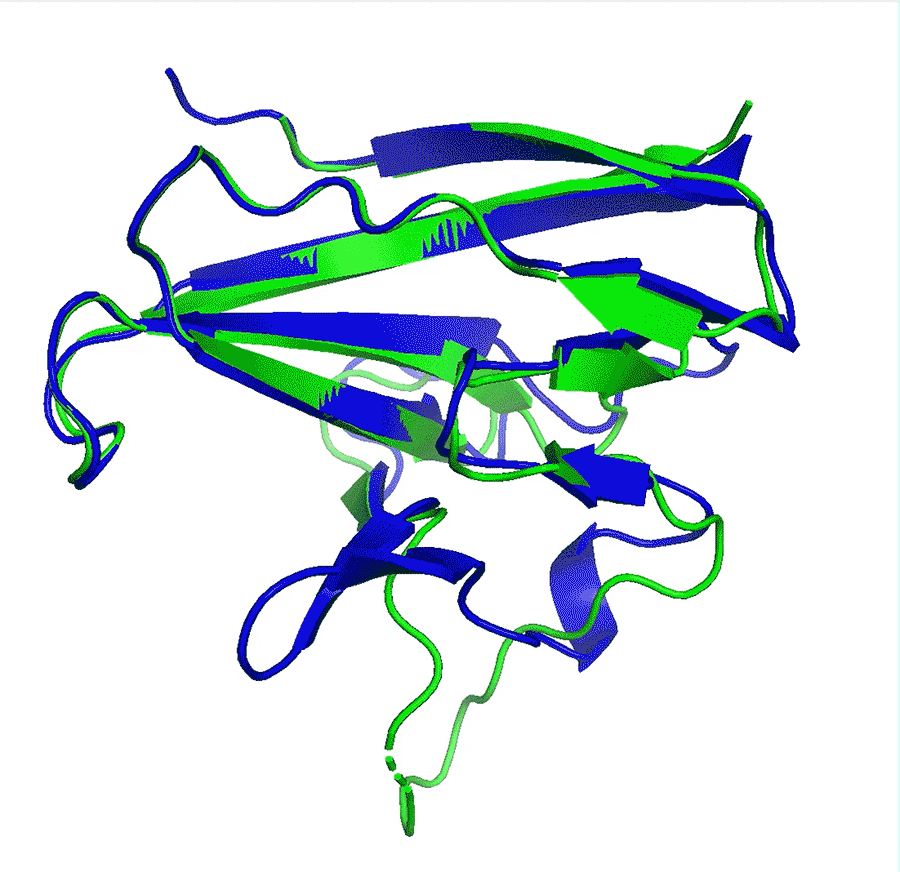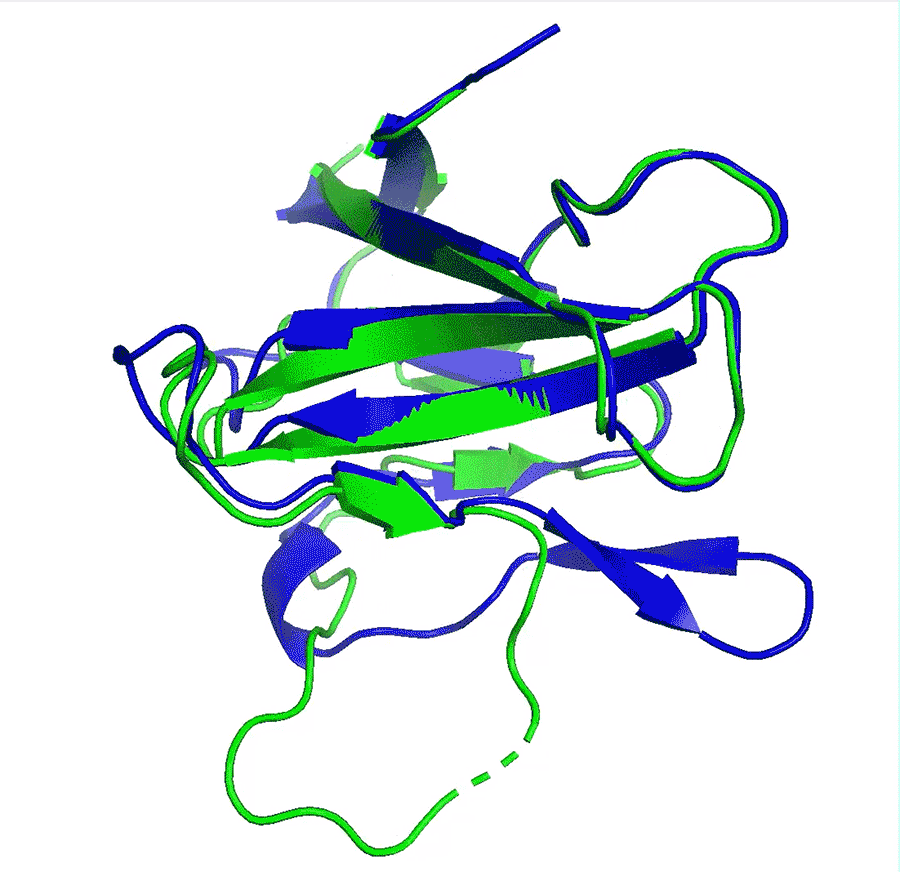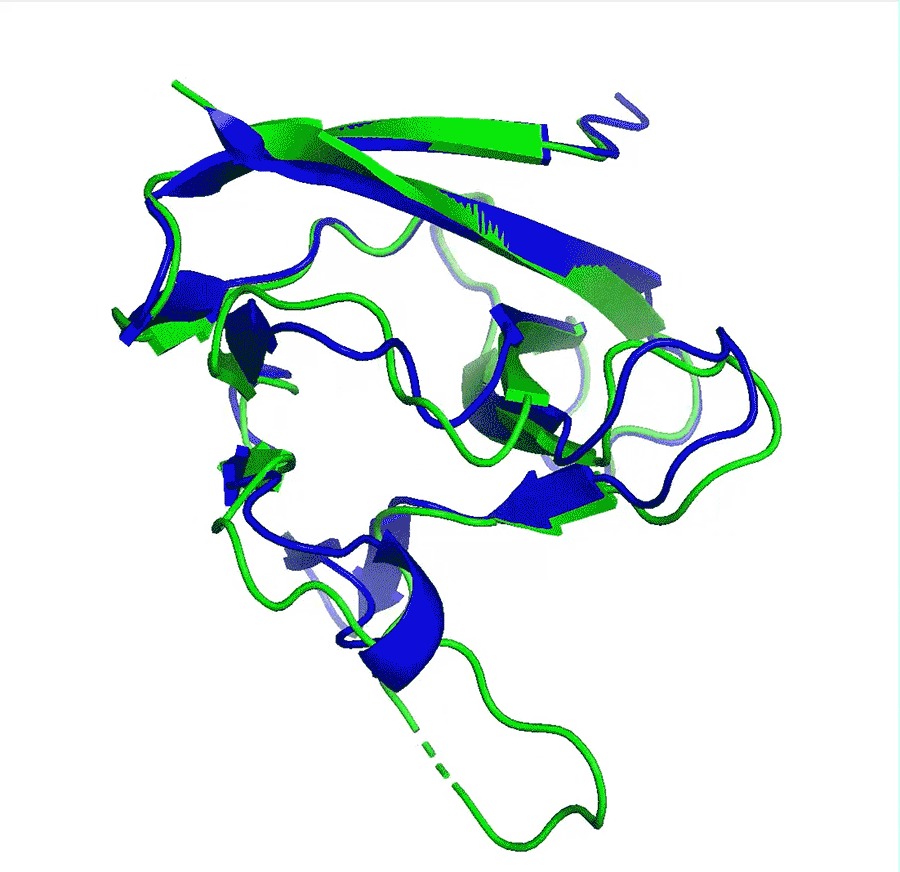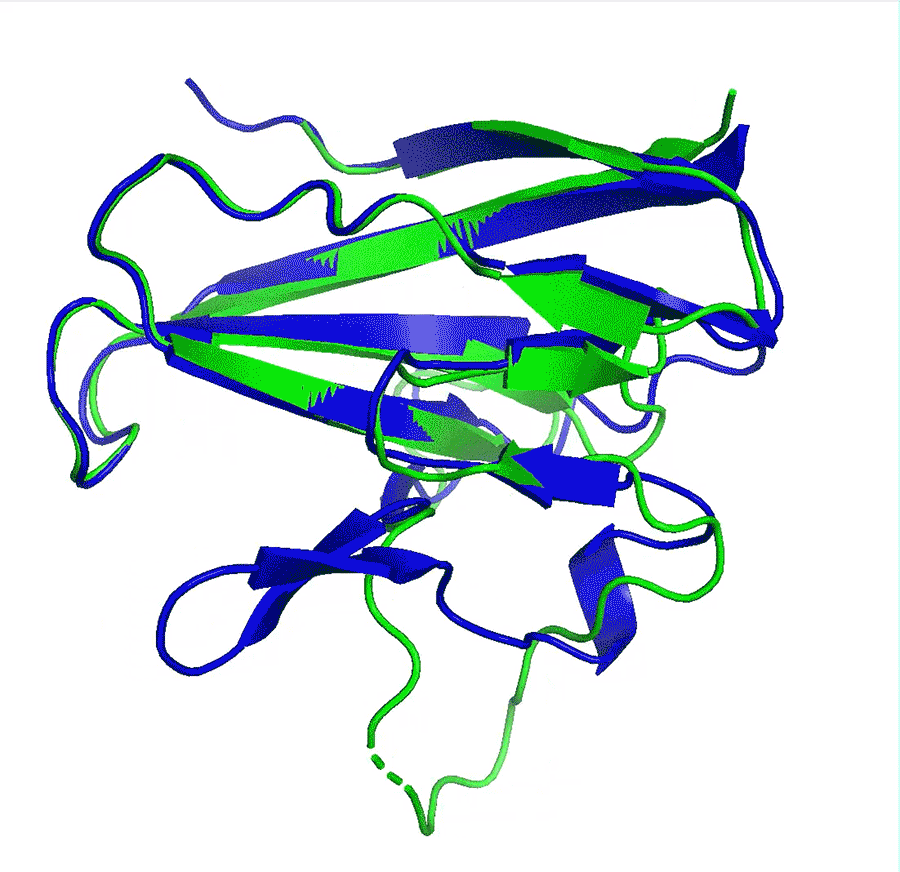
Deep-learning network AlphaFold2’s perfomance at predicting how proteins fold has caused great excitement in the scientific community
A team at Google offshoot DeepMind say that their artificial intelligence (AI) network has made a huge leap in solving the 50-year-old protein folding problem after it outcompeted all other teams at in a protein structure prediction challenge. The program has been received with enthusiasm by researchers worldwide who say it could revolutionise biology, in particular areas such as drug design or environmental sustainability.