Scientists trying to copy natural muscles have several problems to overcome, Clare Sansom finds
Think for a minute about what your body is doing as you read this. Your eyes scanning the page; your fingers tapping a keyboard or swiping a screen; your hands perhaps holding a paper copy of Chemistry World; your back supporting you as you sit in a chair. Even when doing something as sedentary as reading, our muscles are constantly at work. Behind the scenes, as it were, cardiac muscles are keeping our hearts beating and smooth muscles are performing other involuntary functions, such as digestion. All these actions and more – Usain Bolt running a record-breaking 100 metres, a hummingbird’s wings beating 50 times a second, an elephant stampeding – are powered by muscles with very similar structures and mechanisms of action.
Muscle tissue is extraordinarily versatile, but its structure appears quite simple. It is not surprising, therefore, that the idea of emulating its properties has been around for many generations. The first to examine this seriously was the 17th-century polymath Robert Hooke. He may be best known to physics students through his eponymous law, but he also made significant contributions to the biological sciences: he was the first to view micro-organisms through a microscope, and the first to coin the word ‘cell’. Hooke’s attempts to create a machine for translating force into motion in a similar way to natural muscles used gunpowder to supply the force. ‘I had a way of making artificial muscle to command the strength of 20 men,’ he commented, although the experiments seem to have been abandoned quite quickly.
On a basic level, muscles can be described as biological actuators
Where Hooke used gunpowder to supply the energy for movement, biological muscles use the near-universal biochemical energy source, adenosine triphosphate (ATP). Over the last half-century, biologists and biochemists have worked tirelessly to determine their structure and mechanism, and so provide today’s artificial muscle designers with a template to work from in full molecular detail. This enterprise began with pioneering structural biologist Ken Holmes at the MRC Laboratory of Molecular Biology in Cambridge, UK, where he elucidated the molecular mechanism of muscle contraction.
Metal muscles
The structure of skeletal muscles – also known as ‘voluntary’ muscles as they are under the control of the nervous system – was known at low resolution long before Holmes started studying their molecular make-up. These muscles consist of regularly repeating functional units, or sarcomeres, which gives them a characteristic striated (or banded) appearance under a light microscope. Muscle tissue is composed of bundles of long, fibrous cells, each of which contains many nuclei and many long, parallel myofibrils. In turn, each myofibril is composed of multiple copies of two long proteins, myosin and actin, together with the proteins that bind them together to form thick and thin filaments respectively. Myosin provides the motor for muscle contraction. Very briefly, each myosin molecule has a motor domain comprising two ‘heads’ that can flex from a bent to a straight conformation through the hydrolysis of ATP, and a long, thin tail that lies parallel to neighbouring actin molecules. Perhaps counter-intuitively, myosin and other motor proteins can be thought of, simply, as catalysts of ATP hydrolysis: these motor proteins are enzymes.
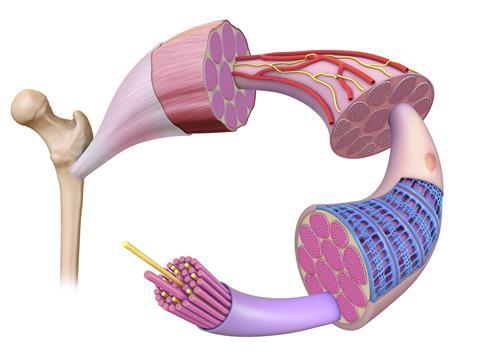
Muscle contraction occurs when the myosin heads extend from the thick filament to grab hold of the neighbouring thin filament and ‘walk along’ it through concerted flexing and extension, hydrolysing enormous numbers of ATP molecules in the process. Interestingly, the myosin heads can only travel along actin filaments in one direction, and each individual muscle can contract and relax but not extend. Their power comes from coordinated action: the simplest action, such as lifting a light object from a table, requires billions of myosin heads to move as one.
On a basic level, muscles can be described as biological actuators. This is the name given to the components of machines that produce and control the movement of a system; scientists and engineers working on artificial muscles often refer to them just as actuators. By definition, any actuator requires both a source of energy and a means of controlling movement through signalling. In the case of skeletal muscles, the source of energy is of course ATP, and the control comes from the nervous system.
Our growing understanding of the complex structure and mechanism of muscle fibres at the molecular level has undoubtedly inspired many groups now working to develop artificial actuators with similar properties to skeletal muscle. However, it is unnecessary to replicate the structure of muscle filaments on – or even near – the molecular level to produce materials with similar, or perhaps just similar enough, properties to be useful. In a recent review, Seyed Mirvakili, chief executive of Seron Electronics in Vancouver, Canada and until recently a postdoc at MIT, has described a wide range of materials including elemental metals, fibres and plastics that have recently formed the basis of such actuators. Many of these materials are quite conventional, simple ones that can be readily bought from a supplier; the properties that enable them to be used as, or in, artificial muscles derive from their structures.
Conducting fibres with cross-sections of tens of nanometres or less, known as nanowires, are frequently used in actuators. These can be of any length but are so thin that their properties cannot be described by conventional mechanics alone, and they have been referred to as quantum fibres. Their usefulness as actuators derives from the fact that they can produce motion in response to light, or to electric or magnetic fields. Much of Mirkavelli’s own research into actuators in drug delivery systems has involved fibres made from niobium-based nanowires. The properties of this ductile transition metal make it ideally suited to clinical use: it is extremely conductive, chemically inert and so extremely safe for clinical use, and relatively readily available. ‘Niobium is not a rare earth, exotic or even particularly expensive,’ says Mirkavelli. ‘It is often used in more conventional medical devices, and some countries have used it on coins because it generates a beautiful surface colour when oxidised.’ These fibres are about 100 times more conductive than carbon nanotubes, which have been used in similar devices; they also have very high capacitance, which means that they can also be used to generate very high magnetic fields for MRI machines.
Soft power
Yves Perriard, head of the Center for Artificial Muscles at the Ecole Polytechnique Fédérale de Lausanne in Switzerland, and his team are developing so-called ‘soft actuators’ from malleable, electro-active polymers for clinical applications. Their dielectric elastomer actuator (DEA) is a hyper-elastic silicone-based polymer sandwiched between two high-voltage carbon-based electrodes and insulated. ‘The process we use to manufacture DEAs is so sensitive that we have to start again if even a single piece of dust gets in,’ he explains.
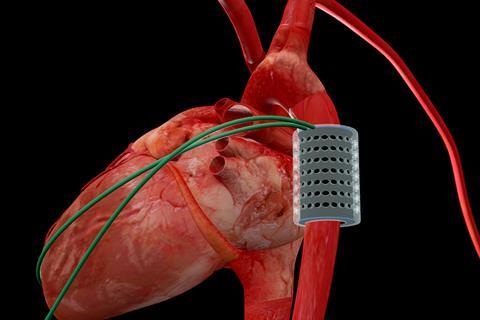
The furthest developed of these soft actuators is an artificial tubular muscle designed to wrap around the aorta – the main artery that carries blood away from the heart towards the body – and contract rhythmically in response to an electrical signal. Therefore, it would squeeze the blood vessel, forcing blood into the heart and so augmenting the aorta’s function. Since Christiaan Barnard performed the first successful heart transplant in South Africa in 1967, more than 50,000 patients have received a donor heart and the average recipient now survives about 15 years after the transplant. This procedure, however, is invasive, expensive and severely limited by the number of donor hearts available: even before the Covid pandemic, patients in the UK frequently had to wait several years for a transplant. The artificial muscle being developed by Perriard and his team is designed to support a failing heart, delaying or even preventing the need for a transplant. ‘Our device has another significant advantage in that it sits around the aorta and so doesn’t enter the heart chambers or the bloodstream,’ says Perriard. ‘The operation is therefore less invasive than open heart surgery, and patients don’t need to take anticoagulants to prevent blood from clotting around the device.’
In a four-hour operation in April 2021, a tubular actuator was positioned on the aorta of a living pig. This maintained the pig’s heartbeat throughout the operation, allowing it to pump blood more efficiently. ‘The pig aorta has a similar diameter to that of humans, and its blood pressure range is similar, so the animal is close to being an ideal model for our experiments,’ says Perriard. ‘However, the ascending aorta – the part nearest the heart, where an artificial muscle would need to be positioned to provide most additional energy – is not long enough to fit one; a calf would be a closer model in this respect.’ Clinical trials are still a few years away, but the success of the first animal trials has unlocked a further CHF 8 million (£6.5 million) in grant funding from the Werner Siemens Foundation to the Center for Artificial Muscles. And Perriard’s group is beginning to develop soft actuators for two further clinical applications: to support urethra function in patients suffering from urinary incontinence, and to restore facial expressions after strokes.
Insect inspiration
Dielectric elastomer actuators may be difficult to make, but they are at least large enough to be tractable. Hongqiang Wang from the Southern University of Science and Technology (SUSTech) in Shengzhen, China, and his co-workers are developing meso-scale actuators inspired by biological muscles as components for insect-sized robots. Conventional electromagnetic motors are relatively heavy, unwieldy and difficult to incorporate into machinery on this scale; in contrast, insect muscles have evolved over more than half a billion years to be robust, lightweight and malleable. On the molecular scale, insect muscles are remarkably similar to vertebrate ones. ‘These miniature actuators are designed in a hierarchical way that mimics biological muscles, with arrays of miniature electrodes taking the place of the actin and myosin filaments,’ explains Wang.
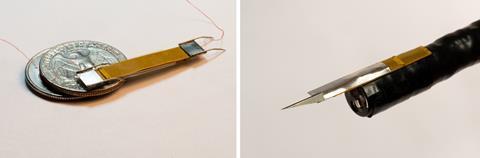
The group at SUSTech have developed two very different miniature robots as case studies to illustrate the versatility of their ideas: an earthworm-like crawling tool, and a cutting tool that could be used in surgery. The first has a very small cross-section that enables it to crawl through narrow gaps and can be used for inspection or search and rescue in toxic environments. ‘Our robot may be thin and flexible, but it is surprisingly robust, more so than other tools with similar uses,’ adds Wang. ‘It can even recover if you step on it.’ The cutting tool could be used for surgery to remove tumours and take tissue samples for biopsies very accurately and precisely. Other potential applications would need robots that can behave like flying ants, for example, and this is only likely to be feasible because the actuators they incorporate are so light and flexible.
It is clearly possible to design materials that have the functional properties of muscles but almost none of their structural ones, at least on the microscopic level; out of the actuators described here, those in Wang’s insect-like robots are the only ones that share any significant features with the microstructure of skeletal muscle. As we learn even more about the mechanism of action of muscle proteins, it is likely that actuators will be designed to emulate their structure more closely. And myosin is only one of a family of motor proteins that convert the chemical energy stored in ATP into movement. Kinesin and the larger, more complex dynein are homologous proteins that use head domains to ‘walk’ in opposite directions along microtubules in a cell’s cytoskeleton, taking organelles and macromolecular complexes with them as ‘cargo’. Kinesin has a key role in mitosis, or cell division, moving cellular components to the opposite ends of a cell before it divides; there are several classes of dynein molecules with varying functions, including the motion of egg and sperm cells. Some viruses, including HIV, hijack dynein to transport themselves towards the nuclei of infected cells.
Kinesin and dynein structures have been determined with x-ray crystallography, the technique that Holmes used to obtain the early actin and myosin structures, but more progress has been made recently using electron microscopy. ‘The “resolution revolution” in electron microscopy during the last few years has enabled us to visualise dynein molecules attached to their microtubule tracks,’ says Anthony Roberts, a structural biologist at Birkbeck College. Emulating these motor proteins, and, perhaps, a material with the properties of the spring-like titin – the longest and largest human protein, which is responsible for the elasticity of muscle tissue – with novel materials might provide future challenges for developers of artificial muscles.
Clare Sansom is a science writer in Cambridge, UK











No comments yet