Chemical bonding
The latest chemistry news and research on bonding, including molecular orbital theory, ionic and covalent bonding and hydrogen bonds, from the Royal Society of Chemistry's magazine, Chemistry World
-
 Research
ResearchUnexpected stability theorised in positron-bound beryllium dimers
Simulations challenge conventional ideas about positronic interactions
-
 Research
Research‘Plumbyne’ compound featuring multiple carbon–lead bonds synthesised
Relatively weak π bonding in the structure opens up possibility for various chemical reactions
-
 Research
ResearchParamagnetic NMR used to probe covalent character of halogen bonds
Assessing hyperfine shifts in paramagnetic NMR spectra provides new details on the covalent character of halogen bonded cocrystals
-
 Research
ResearchWater squeezed into 2D channels conducts electricity 100,000 times better
Network of quasi-2D hydrogen bonding may be responsible for effect
-
 Research
ResearchElectron irradiation converts hydrocarbon crystals into nanodiamonds
Flawless nanodiamond synthesis that wasn’t thought possible accomplished by transforming adamantane
-
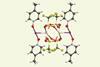 Research
ResearchDiscovery of unusual iodine–silver bond opens up new possibilities for coordination chemistry
Crystallographic studies reveal that the bond is similar in length to typical metal–metal bonds
-
 Research
ResearchNobelium becomes heaviest element with identified compounds
Complexes containing hydroxide, water and dinitrogen ligands detected as researchers probe chemistry on the edge of the actinide series
-
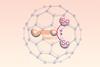 Research
ResearchLanthanide–carbon triple bond synthesised and characterised
Cluster stabilised within a C80 fullerene cage
-
 Research
ResearchActinide bonding could be tweaked by adjusting oxidation states
Modelling reveals that control of mysterious phi bonds could change behaviour of f-block elements
-
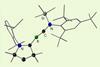 Research
ResearchCarbon–boron triple bond formed for the first time in a neutral novel molecule
Synthesis could help chemists better understand bonding
-
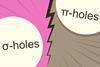 Research
ResearchComputational study says nucleophile finds σ-holes more attractive than π-holes
A σ-hole leads to a stronger bond with NH3 than a π-hole on the same atom
-
 Opinion
OpinionLetters: February 2025
A reader argues for greater use of quantum chemistry in Iupac definitions
-
 Opinion
OpinionA high-pressure insight into the structure of water
The hydrogen-bonded network in liquid water resists compression; density increases instead arise from molecules moving into voids
-
 News
NewsBeyond hydrogen bonding: new definitions for secondary bonding interactions to end confusion
The 20-year struggle to define secondary bonding interactions
-
 Webinar
WebinarCopper-catalysed carbon-heteroatom bond-forming processes
Explore cutting edge copper-catalysed carbon-heteroatom bond formations used in industrial applications
-
 News
NewsRobert Mulliken’s Nobel prize medal latest to go up for auction
Mulliken won the Nobel prize in chemistry in 1966 for developing molecular orbital theory
-

-
 Feature
FeatureIlluminating antiaromaticity
Aromaticity’s dark alter-ego is ready to emerge into the sunlight. James Mitchell Crow talks to the scientists trying to exploit the instability
-
 Webinar
WebinarLab XAS: a new tool for materials characterisation
Explore the utility of lab XAS as an everyday tool for materials characterisation
-
 Opinion
OpinionBonds are the ties that bind chemistry
Those seemingly simple sticks belie our most complex concept