Chemical bonding – Page 3
-
 Research
ResearchTextbook electronegativity model fails when it comes to carbon–halogen bond strengths
Computational analysis finds that it’s size, not electronegativity differences, determining bond strength within periodic table groups
-
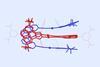 Research
ResearchClippanes join rotaxanes and catenanes in mechanically interlocked molecule family
Keck-clip molecules consist of two entangled gold–carbene metallotweezers
-
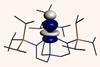 Research
ResearchUranium’s strong covalent bond breaks periodic table predictions
Actinide’s unusual covalency could explain its ability to fix nitrogen
-
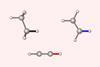 Research
ResearchChemists reconsider C–H and C–C bond length rationale
Quantitative proof for steric repulsion theory
-
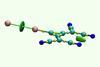 Research
ResearchAnti-electrostatic halogen bonding shown in solution for the first time
UV-vis spectroscopy and computational analysis explore nature of counterintuitive anionic interaction
-
 Research
ResearchBond between artificial and natural atom measured for the first time
Chemists reveal the tiny forces in chemical bonds between quantum corrals and AFM tip atoms
-
 Opinion
OpinionHow values influence decisions in science
Empirical evidence is not always sufficient to determine the models we use
-
 Research
ResearchExotic bond takes computational chemists by surprise
New results could help to solve the dispute around the strange Na–B bond in NaBH3–
-
 Research
ResearchUnstable silicon double bonds tamed in polymers made on metals
Process could ‘open the door’ to new electronic materials
-
 Opinion
OpinionHow philosophy helps solve puzzles in chemistry
Without realising it, chemists use a range of philosophical tools to probe the world
-
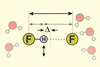 Opinion
OpinionWhen does a hydrogen bond become a covalent bond?
Ultrafast infrared spectroscopy probes the character of the short, strong bonds in HF2–
-
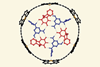 Research
ResearchIntricate supramolecular rosette demonstrates power of cooperative interactions
Rosette assembles inside porphyrin nanoring when all components are present
-
 Research
ResearchHydrogen bonds join forces to maximise water–water interaction
Cooperative environment encourages stronger chemistry
-
 Research
ResearchSimulation says supercritical water has no hydrogen bonds
Computational approach seeks to clarify bonding confusion
-
 Research
ResearchHydrogen bond imparts more stability on transition state than expected
Study reveals importance of repulsive interactions
-
 Research
ResearchMacrocycles slide freely across carbon nanotubes
Simulations suggest macrocycles show superlubricity at room temperature
-
 Research
ResearchExcited state potential energy curves reignite diatomic carbon’s bond order conundrum
Molecular orbital theory-based approach backs quadruple bond
-
 Research
ResearchBeautiful molecules ring up with a purpose
How Stephen Goldup used his experience in organic synthesis to find a supramolecular niche
-
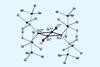 Research
ResearchNew silicon–silicon bond is a rare example of a π bond without a σ bond
Discovery made in silicon analogue of bicyclobutane
-
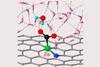 Research
ResearchHydrogen bonds are single atom nickel’s secret for carbon dioxide reduction
Computational calculations show that hydrogen atoms and charge capacity are behind nickel’s high activity and selectivity in electrochemical carbon dioxide reduction