Quantum tunnelling is the dominant process driving chloride ions to leach from polyvinyl chloride (PVC), a computational study concludes.
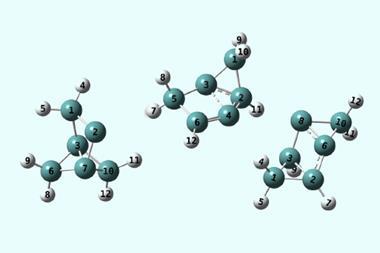
Gbolagade Olajide and Tibor Szilvási of the University of Alabama, US, made the surprising discovery while studying a base-assisted E2 reaction in which a chloride ion is liberated from a PVC polymer chain. ‘We did some exploratory calculations, then my student [Olajide] came back to me that, okay, this is the barrier, this is the energetics, and I see that the barrier is extremely narrow,’ says Szilvási.
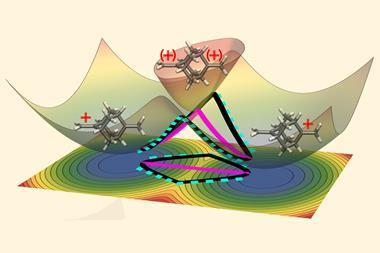
This was the first hint of a phenomenon called quantum tunnelling. In contrast to thermal activation, where reactants gain sufficient energy to jump the energy barrier of a reaction, the wave-like nature of electrons and nuclei means they can also seep through the barrier without possessing the requisite energy. But since the barrier weakens the wavefunctions exponentially, it must be narrow for tunnelling to be a realistic prospect.
Although he saw this slender barrier, Szilvási was still sceptical. ‘I was just immediately thinking that this should not be the case. If you have chlorine, which is a really heavy atom, involved in a reaction, then normally there is no tunnelling at all.’
But further probing only strengthened the case. ‘Everything still pointed to the same direction,’ says Szilvási. ‘Then we decided that we will do a really detailed study on this … and that also showed the same answer.’
The researchers used density functional theory (DFT), a widely used technique in computational chemistry, with less common add-ons to model tunnelling. By switching the tunnelling contributions on and off, they were able to compare the reaction rate that arose from tunnelling to that from thermal activation alone. They were astonished to find that in 93% of reactions at room temperature, the reactants burrowed through the barrier rather than jumping it.
Further calculations, in which they replaced various atoms by isotopes of different mass, pinpointed that hydrogen was leading the charge in this quantum escape. This might be expected, being the lightest element, but Szilvási makes the intriguing suggestion that ‘somehow it drags the chlorine with it’.
Peter Schreiner, an organic chemist at Justus Liebig University Giessen, Germany, is similarly struck by the results. ‘I was surprised that something as common and mundane as PVC would be affected by tunnelling,’ he says, adding that it is the first time the effect has been demonstrated for a polymer.
Including quantum tunnelling in DFT calculations is costly in computer time, and thus tempting to dismiss as an exotic folly. Schreiner, who has long argued that tunnelling’s importance is overlooked, is thrilled that the researchers have found such a stark example in an everyday reaction.
‘I would say [quantum tunnelling is] alien to most people, and they think, oh it’s probably not relevant to me,’ he says. ‘These authors have opened the gate for some real-time application – that is, how to get rid of certain plastics.’
PVC is the world’s third-most produced plastic, used in everything from vinyl records and window frames to rainwear and inflatables. It is also a common choice for modern water pipes, and many old lead pipes are being replaced by PVC. Szilvási and Schreiner both highlight the issue of chloride leaching from underground pipes into the environment, particularly if buried in alkaline soil. Conversely, if PVC is to be recycled, the efficient removal of chloride and other additives is essential.
Szilvási cautions that while his work may explain chloride leaching, it doesn’t offer a magic bullet to prevent it. ‘Quantum tunnelling will always be there, and you cannot avoid it.’
References
G Olajide and T Szilvási, Chem. Commun., 2024, DOI: 10.1039/d4cc03489a















No comments yet