All articles by Chris Nawrat – Page 2
-
-
-
-
-
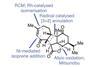 Opinion
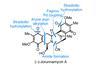
Opinion(‒)-Pavidolide B
An innovative approach to making five-membered carbon rings makes for a strikingly short synthesis
-
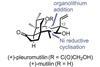 Opinion
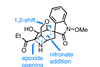
Opinion(+)-Pleuromutilin
Total synthesis is sometimes the only way to explore the chemical space around a natural product
-
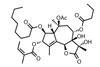
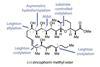 Opinion
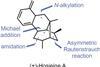
Opinion(+)-Zincophorin methyl ester
New and old reactions combine for an elegant and concise synthesis
-
-
-
 Opinion
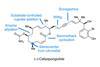
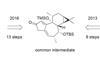
Opinion6-epi-Ophiobolin N
When it comes to cascade reactions, radicals are king of the ring-formers
-
-
-
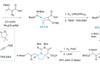 Opinion
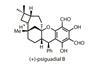
OpinionBatzelladine B
Taming basic and reactive nitrogen atoms makes alkaloids more attractive targets, says BRSM
-
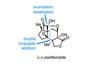 Opinion
Opinion(–)-Jiadifenolide
BRSM wonders what makes a route so good it becomes the last total synthesis of a complex target
-
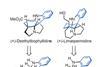 Opinion
OpinionLimaspermidine and deethylibophyllidine
Desymmetrisation offers a neat way to add tricky features, says BRSM
-
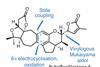 Opinion
OpinionRubriflordilactone A
Sometimes it’s worth building aromatics instead of buying them, says BRSM
-
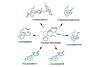 Opinion
OpinionLycopodium alkaloids
Not all natural products are created equal. BRSM looks at a flexible route to some perennial favourites
-
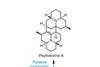 Opinion
OpinionPsylloborine A
Late stage dimerisation is a tantalisingly elegant but risky strategy for total synthesis, says BRSM
-
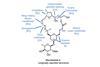 Opinion
OpinionMandelalide A
New reactions need to belong in a synthesis, says BRSM, not be forced in for show
-
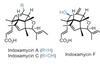 Opinion
OpinionIndoxamycins A, C and F
BRSM gets to the core of a divergent synthesis of this natural product family
- Previous Page
- Page1
- Page2
- Next Page