This year’s chemistry Nobel prize was awarded to three US-based scientists: Moungi Bawendi at the Massachusetts Institute of Technology, Louis Brus at Columbia University, and Alexei Ekimov who was most recently chief scientist at Nanocrystals Technology, having previously worked at the Vavilov State Optical Institute in St Petersburg, Russia. The award was given for their ‘discovery and synthesis of quantum dots’ – but what are quantum dots, and why are they worthy of a Nobel prize?
What is a quantum dot?
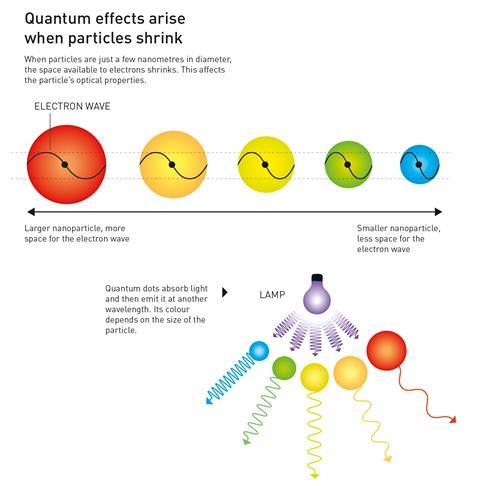
Quantum dots are tiny crystals of semiconducting material. The crystals are so small – just a few nanometers wide – that their physical size actually confines the electrons in the material to the point that it changes their behaviour. This means that quantum phenomena determine the properties of these tiny crystals – hence the name quantum dots.
The extent of the confinement depends upon the size of the particle, so changing the size of the particle changes its properties in a predictable way. This effect can be used to tune the band gap of the semiconductor so that dots emit different colours of light when illuminated by a single-wavelength source – smaller dots will emit blue light, while larger ones emit at the red end of the visible spectrum. Their melting points and their ability to transfer electrons can also be tweaked in this way.
Like other semiconductors, quantum dots tend to be made of combinations of transition metal and metalloid elements. Cadmium selenide and cadmium telluride are among the most regularly used materials.
So quantum dots are nanoparticles?
Yes. But not every nanoparticle is a quantum dot. Only some materials (such as semiconductors) will show quantum size effects in their electronic structure when they are made into nanoscale particles.
Why did they win a Nobel?
From a purely fundamental point of view, quantum dots are remarkable substances that bridge the gap between matter at the atomic and molecular scale and bulk matter. And thanks to their highly tuneable optical and electronic properties, quantum dots have found application in a whole array of technologies.
One of their most widely known uses is in forming the basis QLED television screens where dots of different sizes are excited by blue light and then emit pure red and green light to give the three-colour output of the pixels in a TV screen. But they also have uses in biotechnology, catalysis, sensors, solar cells and more. In particular, the Nobel committee highlighted the use of quantum dots in medical devices that are used to map biological tissue because the dots’ fluorescence is brighter and longer-lived than that of other fluorescent tags such as molecular fluorophores. This means they can help to guide surgeons when removing tumours, for example.
In 2021, global sales of quantum dots were worth around $4 billion (£3.3 billion), a figure that is predicted to double by 2026.
From a chemistry perspective, quantum dots have been investigated for all sorts of different applications – from converting heat into electricity to producing light-emitting 3D printer ink. They are also widely researched photocatalysts that can even, in some cases, catalyse carbon–carbon bond forming reactions more efficiently than precious metal catalysts.
What did the laureates do?
Researchers had predicted since the 1930s that size-dependent quantum effects should be observed in particles that are a few nanometres in size. But at the time there was no way to controllably produce materials on this tiny scale. As the 20th century progressed, the first observations of the effect were made in thin films and on the surface of bulk materials – the share of the Nobel prize in physics awarded to Zhores Alferov and Herbert Kroemer in 2000 recognised their work on these sorts of semiconductor heterostructures.
In the early 1980s, Alexei Ekimov first identified quantum size effects in nanoparticles, while working at the Vavilov State Optical Institute in St Petersburg. He was carrying out experiments on doping glass with copper chloride. By varying the temperature and speed at which the glass was formed, Ekimov’s team could control the size of copper chloride crystals that formed within the glass matrix. During x-ray experiments on these materials, he noticed that the wavelength of copper chloride absorption lines varied depending on the size of crystals, which Ekimov attributed to quantum size effects.
Meanwhile, Louis Brus was working on colloidal systems where semiconducting materials were suspended in liquids. In 1983, Brus observed quantum size effects in his experiments with cadmium sulfide particles, noting that the optical properties of colloidal CdS had changed overnight because the crystals had dissolved and then precipitated out as crystals about three times larger. Spectroscopic analyses revealed differences between the absorption behaviour of the two sets of crystals – showing the influence of quantum-size effects.
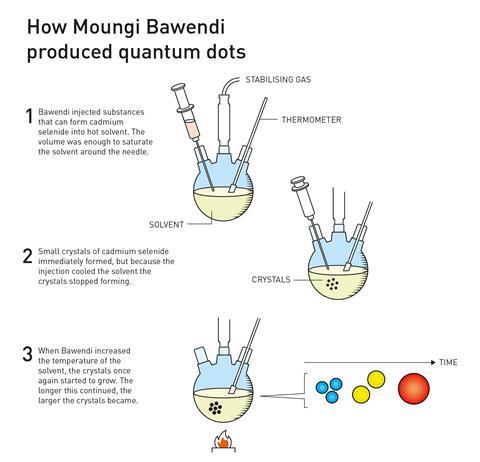
In 1993, Bawendi developed a means to produce cadmium selenide crystals with near perfect control over their size. The method involved injecting organometallic precursors into a hot solvent. This ‘hot injection’ technique gave the team control over the crystal nucleation point because the solution becomes supersaturated very rapidly, which produced quantum dots with almost uniform size. This discovery opened the door to using quantum dots in real-world applications.
What about the leak?
There is often controversy around the Nobel prizes, usually surrounding researchers who some believe were overlooked for the award. But this year a major talking point surrounds the apparent leak of the winners’ names hours before the official announcement.
The selection of the winners of the Nobel prizes is usually shrouded in secrecy. But this year, the Swedish broadcaster SVT reported that it had received a press release from the Royal Swedish Academy of Sciences naming the winners more than four hours before the official announcement. It is the first time that the winners of the chemistry prize have been leaked ahead of a Nobel announcement. It appears the email was sent inadvertently, and will no-doubt have caused headaches for the academy.
















1 Reader's comment