From the law of octaves to the periodic table as we know it, Mike Sutton traces how chemists put their house in order
A century and a half ago, chemists were struggling to organise their ever-growing list of elements into meaningful patterns. In 1864, one of them took an important step by leaving a blank space in his system for an undiscovered element. At first his ideas were dismissed as idle speculations, but after the hypothetical element was discovered his contribution was belatedly acknowledged.
That chemist was John Newlands. Born in London, UK, in 1837, he was educated mainly at home by his father, a Presbyterian minister. In 1856, John enrolled at London’s Royal College of Chemistry, and a year later was employed as an assistant chemist at the Royal Agricultural Society. But in 1860 there was a dramatic break in his career.

His mother, Mary Newlands (née Reina) was Italian, and John supported the struggle for a unified independent Italy. He served for some months with Giuseppe Garibaldi’s volunteer army, returning to London after it was disbanded. In 1862, he married Jane Rickings, and by the mid-1860s was working as a freelance analytical chemist, teaching at colleges and schools and submitting articles to William Crookes’ Chemical News.
Newlands’ papers addressed a long-running debate. For half a century, chemists had been seeking a rational classification system for the elements, analogous to those which 18th-century natural philosophers had developed for animals, plants and minerals. A satisfactory plan had not yet emerged, despite some promising developments.
Rise of the triads
An early breakthrough came in 1817 when German chemist Johann Döbereiner noticed the several clusters of three elements possessing remarkably similar chemical and physical properties: calcium, strontium, barium; lithium, sodium, potassium; and chlorine, bromine, iodine. By the mid-19th century, chemists had recognised more of these ‘triads’ and some were already constructing patterns involving larger groups of elements.

Among the most active in this search were the Germans Leopold Gmelin and Max Pettenkofer, the Frenchman Jean Baptiste Dumas, and England’s William Odling and John Hall Gladstone. They all made some progress, but none produced a fully satisfactory system. All of them were handicapped by continuing uncertainty about the atomic weights (in modern terms, the relative atomic masses) of many of the elements.
Chemists had long known that hydrogen and oxygen combine to form water in a ratio of (almost) one to eight by weight. Oxygen, therefore, was said to have an ‘equivalent’ of eight. If water’s formula were HO, as many then believed, then the atomic weight of oxygen would also be eight. But some chemists insisted that water was H2 O, making oxygen’s atomic weight 16. Similar disagreements over the formulae of other compounds caused further uncertainty.
Nevertheless, a few investigators (including Jacob Berzelius and Charles Gerhardt) continued the search for reliable atomic weights. Other data sources – Gay-Lussac’s law of combining volumes, Mitscherlich’s law of isomorphism, the atomic heat equation of Dulong and Petit – gave them some guidance. But many chemists mistrusted these calculations, preferring to stick to the empirically determined equivalents. And there were some (for example, Benjamin Brodie and Marcellin Berthelot) who rejected the atomic theory entirely.
In September 1860, the Karlsruhe Congress ended with Europe’s chemists still disagreeing over atomic weights. Before departing, however, Stanislao Cannizzaro distributed a paper which impressed several participants who read it on the journey home. And so, while Newlands fought in Italy’s Risorgimento, a revolution in chemical theory was launched in Germany – ironically, by an Italian who had participated in the Sicilian revolt of 1848.
First tables
Cannizzaro revived the hypothesis suggested in 1811 by Amedeo Avogadro, that equal volumes of gases (under identical conditions of temperature and pressure) contain equal numbers of molecules. Some chemists misunderstood Avogadro’s proposal because his terminology blurred the distinction between molecules and atoms. Others objected because his theory implied that some common elementary gases existed as diatomic molecules.
Molecular formulae like H2, O2 and N2 were mistrusted by scientists who believed that molecules were bound together by attraction between opposite electrical charges. But this dualistic theory of chemical bonding was less popular by the time Cannizzaro revived Avogadro’s hypothesis, and many chemists were attracted by his promise of a better way of calculating atomic weights.
Since two volumes of hydrogen combine with one volume of oxygen (measured under similar conditions), Cannizzaro accepted the H2 O formula for water and an atomic weight of 16 for oxygen. Although some atomic weights remained difficult to measure precisely, many could now be determined with greater confidence. This encouraged scientists to arrange the elements in order of increasing atomic weight and look for recurring patterns in their properties.
Among the boldest was French mineralogist Alexandre de Chancourtois. He placed the elements along a spiral line running around the outside of a cylinder, which put at least some groups of similar elements into vertical sequences. But his 1862 paper attracted little interest, as it was printed without a diagram, making his argument hard to follow – and a more fundamental flaw was that his list of ‘elements’ included compounds and alloys.
Newlands arranged the elements in a grid structure, which reflected their chemical similarities
In 1863, Newlands’ first paper simply sorted the elements into 11 groups. Some were familiar chemical families, like the alkali metals and the halogens, but others were more heterogeneous. He also listed arithmetical relationships between the equivalents of various elements, noting in particular that the equivalents of chemically similar elements often differed by eight, or a multiple of eight.
Newlands employed the old equivalents here ‘for the sake of perspicuity’. In July 1864, however, his next paper used Cannizzaro’s atomic weights, which were becoming better known in Britain through the advocacy of Alexander Williamson. This time Newlands focused on a smaller number of elements, including only ‘those elementary groups the existence of which is almost universally acknowledged’.
These he arranged in a grid structure, which reflected chemical similarities between the elements, while also taking account of the ratios of their atomic weights. Lithium and magnesium were given multiple positions, as they fitted arithmetically into more than one triad. Alongside its omissions and errors, this table contained two significant innovations.
Firstly, it placed tellurium (128) before iodine (127), disregarding the general principle of ordering elements by atomic weight. Newlands argued that tellurium’s chemical similarities with sulfur and selenium, and iodine’s with chlorine and bromine, made this anomaly unavoidable. Its cause was not understood until Frederick Soddy, Henry Moseley and James Chadwick alerted chemists to the existence of isotopes, atomic numbers and neutrons decades later.
Its second innovation was a gap left for an undiscovered element between silicon and tin. Others may have speculated that pairs of similar elements might be incomplete triads, but Newlands seems to have been the first to incorporate such a proposal in a larger grid of elements.
Spanning the octaves?
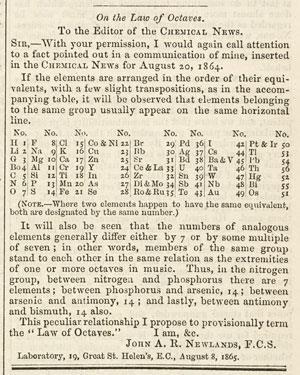
Newlands’ third table appeared in August 1864. It was even briefer, and still contained some misplaced elements, but it introduced another important development – tagging the elements with consecutive ordinal numbers. This was an important step towards a periodic law, but Newlands gave his critics an easy target by linking it with an unfortunate analogy.
‘Here the difference between the number of the lowest member of a group and that immediately above it is seven,’ he wrote. ‘In other words, the eighth element starting from a given one is a kind of repetition of the first, like the eighth note of an octave in music.’
His fourth paper, published in 1865, formalised this suggestion as the ‘law of octaves’. In his enlarged table of around 60 elements, Newlands’ octave sequence worked well for the lighter elements. But to maintain it beyond them he had to place several elements alongside uncongenial neighbours, while making some pairs (like cobalt and nickel, and lanthanum and cerium) share the same number.
A meeting in London of the Chemical Society in 1866 gave Newlands’ octaves an unsympathetic reception – one member asked sardonically if he had considered arranging the elements in alphabetical order. The society’s journal rejected the paper, and William Odling (who had chaired the meeting) justified the decision by stating that the society’s rules prevented it publishing works of a purely theoretical nature.
There were fewer inhibitions at the Quarterly Journal of Science, where Odling’s own equally speculative table of elements had appeared in October 1864. Like Newlands, Odling noted arithmetical regularities in atomic weights and left gaps for undiscovered elements, though there were significant differences between their systems.
Odling had a long-standing interest in this topic, and there are no grounds for accusing him of plagiarism. However, Newlands withdrew from the debate. In 1868, he became the chief chemist at a London sugar refinery, and subsequently patented several improvements to its processes. Octaves seemed to have dropped from the agenda when Mendeleev’s table of elements appeared in 1869.

Enter Mendeleev
Dmitri Mendeleev was the son of a Russian schoolteacher in Tobolsk, Siberia. When blindness compelled his father to retire, Dmitri’s mother funded the boy’s education by taking over and running a local glass factory. In 1850, she brought him to St Petersburg, where he qualified as a schoolteacher. After completing a Master’s degree in 1856, he taught chemistry at St Petersburg University and in 1867, having recently gained his doctorate, he became a full professor.

Following the Karlsruhe Congress, Mendeleev recognised that atomic weights were the key to classifying the elements. He became fully engaged with this task in 1868 while compiling a general textbook, later writing in an English edition ‘I began to look about and write down the elements with their atomic weights and typical properties, analogous elements and like atomic weights on separate cards, and this soon convinced me that the properties of the elements are in periodic dependence upon their atomic weights. I have never once doubted the universality of this law, because it could not possibly be the result of chance.’
Mendeleev often laid out these cards as if playing a game of patience, always seeking possible patterns. Like Newlands (of whose work he was then unaware), he saw the need to leave gaps for undiscovered elements. His first table appeared in a Russian journal in 1869, reaching a wider audience through a German abstract. Meanwhile in Germany, Lothar Meyer had independently reached similar conclusions.
Meyer, the son and grandson of physicians, was destined for a medical career until chemistry seduced him. After studying at various German universities he completed a doctorate at Breslau in 1859, and attended the Karlsruhe Congress in 1860. Like Mendeleev, he became involved with the problem of classifying the elements while writing a textbook. If its publisher had not been sluggish, his table would have appeared before Mendeleev’s.

Meyer was particularly interested in physical parameters like conductivity, melting point and atomic volume (density × atomic weight). He found that when these values were plotted against atomic weights, chemically similar elements occupied the peaks and troughs of the graph, indicating that some periodic function was involved.
Both men recognised that a simple grid could not accommodate the transition metals, and (even more so) the rare earth metals. They therefore added extra groups, or sub-groups, at the heavier end of the table. Both also left gaps for undiscovered elements, to avoid placing known elements in mismatched company.
Mendeleev’s system was not universally welcomed, and its weaker points were rightly challenged. But most chemists became convinced after three of his hypothetical elements were discovered – gallium in 1875, scandium in 1879 and germanium in 1886 – especially as he had accurately predicted their physical and chemical properties. (The non-appearance of several of his other predicted elements was tactfully forgotten.)
In 1882, the Royal Society awarded Mendeleev and Meyer its Davy Medal. Newlands responded by republishing his papers of the 1860s, with a plea for recognition of his contribution. In 1887 – a year after the discovery of the element he had predicted in 1864, germanium – he too received the Davy medal.
As the 19th century ended, the noble gases and the radioactive elements introduced further complications. But the table survived, and the quantum revolution of the 20th century gradually explained its idiosyncrasies. We know now that the rules governing the table’s structure are more complex than the law of octaves that so amused the Chemical Society. And yet many of these rules are expressed in wave equations which make their own silent music – so perhaps the last laugh belongs to Newlands after all.
References
C Giunta, Elements and atoms http://bit.ly/1bgg8Ul
E R Scerri, The periodic table: its story and significance, Oxford University Press, 2007
J W Van Spronsen, The periodic system of chemical elements, Elsevier, 1969
M E Weeks and H M Leicester, Discovery of the elements (7th edn), Journal of Chemical Education, 1967













1 Reader's comment