How do animals survive in the extreme cold? James Mitchell Crow investigates the proteins that do the job
Life can be tough in the icy cold waters of Antarctica. Seawater will dip close to –2°C before it freezes, a temperature that would kill most fish. Yet certain species have adapted to these sub-zero temperatures. Some teleost fish, for example, live happily in the tunnels and holes that form in floating islands of sea ice. Identifying the chemical tricks that these fish play to lower the freezing point of their blood below that of seawater proved no simple task. We now know that the fish deploy small amounts of highly active antifreeze proteins.
Since these proteins were first discovered in Antarctic fish in the late 1960s, many other species have been found to employ similar compounds to avoid the potentially fatal formation of ice crystals in their body at sub-zero temperatures. ‘Fish have it pretty nice,’ says John Duman, a biologist who studies cold adaptation at the University of Notre Dame in Indiana, US. ‘The coldest that a fish will see is –1.9°C – terrestrial organisms will see much, much colder temperatures.’ Duman has studied Alaskan beetles whose larvae can sometimes survive temperatures below –100°C, and has found potently active antifreeze compounds in flora and fauna from frogs to grasses.
Despite the structural diversity of these compounds, the evidence is growing that they might all function through a common mechanism. At the same time, progress is finally being made in applying the knowledge gained from studying natural antifreezes for practical applications, from higher quality frozen foods to the cryopreservation of tissues or blood supplies.
Salty secret
Antifreeze proteins are of such potential commercial interest because they function quite differently to the typical antifreeze currently used to protect car engines, for example. Polyethylene glycol and the like lower the freezing point of water through colligative effects: adding any solute to water raises its entropy, lowering the point at which it will freeze. Dissolved salts have the same effect, which is the reason why seawater has a freezing point close to –2°C.
In the 1960s, the researchers hunting through the blood of Antarctic fish, trying to identify the compounds that would account for its lowered freezing point, assumed that colligative effects must be at play. ‘When people first tried to figure out what polar fish were doing, they were looking for polyols and low molecular weight sorts of antifreezes,’ recalls Duman. ‘They knew their physical chemistry, and knew that a protein wasn’t going to be important because you couldn’t get enough of it into solution to lower the colligative freezing point.’ And yet the compounds isolated could never fully account for the fact that the teleost fish’s blood remained liquid to below –2°C.
Arthur DeVries, an animal biologist specialising in polar fish species, ultimately solved the mystery, publishing his discovery in a 1969 Science paper.1 Based at the time at the University of California, Davis, in the US, DeVries happened to look at proteins in fish blood, and identified an unusual glyoprotein in one of his extracts that he found was responsible for the missing 30% of freezing point depression in teleost fish.
Shortly after his discovery, DeVries moved to the Scripps Institute of Oceanography, where Duman joined his research group as a graduate student. ‘At that point, just one or two labs were working on antifreeze proteins,’ he recalls. Duman started to look for biomolecules with freezing-point-lowering activity in other polar fish, this time in the Arctic. He identified such a protein in an unrelated species called the winter flounder – which had a very different structure to the compound DeVries first identified in the teleost fish, not least in the fact that it lacked any carbohydrate component.2 ‘Since then there have been other fish antifreeze proteins found, again with much different structure,’ Duman says.

When Duman began his independent academic career at Notre Dame, he switched his focus to freeze-avoiding insects, which remain at the centre of his research today. One extensively studied species is an Alaskan beetle, Dendroides canadensis, which produces one of the most potent antifreeze proteins known. ‘The structure is a 12- or 13-mer repeat structures where every sixth residue is a cysteine, disulfide-bridged, and it produces this beta barrel with threonine groups very regularly arranged with hydroxyl groups.’
Even within this single species, Duman and his colleagues have discovered that the beetle produces around 30 different antifreeze protein subtypes, each apparently fine-tuned to function most effectively within different areas of the beetle’s body, from the gut to the haemolymph (blood) to the epidermis.
Protein-free
Since Arthur DeVries’s 1969 discovery that small amounts of a highly active protein could protect polar fish from freezing, the race has been on to identify such proteins in other species. Perhaps that is why the discovery of a different, non-protein, antifreeze too so long.
John Duman at the University of Notre Dame in Indiana, US, and his colleagues hit the problem while searching for the antifreeze compounds in a freeze-tolerant Alaskan beetle called Upis ceramboides.8 ‘We tried to purify the protein for a long time but we couldn’t – the reason why was because it wasn’t a protein,’ says Duman. The compound responsible was in fact a glycolipid.
Since that initial discovery, the team has identified antifreeze glycolipids in one plant species, six insect species and a frog. ‘I think when people look around they will find that glycolipids are common,’ Duman says. Unlike antifreeze proteins, the glycolipid tends to be on cell membranes. ‘Its function, I think, is probably mainly to prevent ice propagation across the cell membrane,’ Duman explains. Rather than attempting to avoid freezing altogether, some species exhibit freeze-tolerance: although their extracellular liquids freeze, they deploy various adaptations – probably including the glycolipids – to prevent ice from propagating across the cell membrane to freeze their intracellular fluids, which would be fatal.
Patterns in diversity
Peter Davies is a biochemist at Queen’s University in Kingston, Ontario, Canada, who studies the structure–function relationship of antifreeze proteins. ‘There is a remarkable difference in their structures – it is pretty clear that they have evolved in different kingdoms, at different times, from different progenitors,’ he says. ‘We see everything from a simple alpha helix to small globular proteins to beta helices.’
Yet despite this diversity, structural themes emerge. Antifreeze proteins typically have one face that is fairly flat, somewhat hydrophobic, often featuring very regular repeating structural motifs across its surface, says Davies. It is this surface that gives the proteins their freezing-point-lowering effects, binding onto the surface of nascent ice crystals and restricting further growth.
DeVries suggested as early as 1971 that antifreeze proteins must work through ice binding.4 The effect becomes apparent when examining the shape of ice crystals grown in the presence of an antifreeze protein. Ice crystals are normally shaped like three-dimensional hexagons, with six ‘prism’ planes around the edge and a ‘basal’ plane on the top and the bottom. Fish antifreeze proteins bind to the prism planes of ice crystals, blocking further water molecules from joining the ice crystal on those faces. Instead, the water has to attach to the basal plane, which is a far slower process.
Davies and his team have carried out site-directed mutagenesis on various antifreeze proteins to demonstrate that it is their flat, hydrophobic face that enables ice binding. The researchers are now closing in on a molecular-level description of the interaction between ice and protein.
‘The mechanism that we think is operational for all these proteins is that they organise waters on the ice binding site, and this bound water looks very ice-like,’ Davies explains. The latest theories about the interface between ice and water suggests that ice crystals have a one-nanometre-thick skin of quasi-liquid water, just two or three water molecules thick. ‘We think the water at the ice-binding site looks very like this quasi-liquid layer. I think these just merge together, and just freeze onto the ice,’ Davies says.5
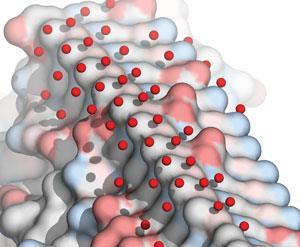
Although this nanoscale quasi-liquid ice layer is impossible to see directly, Davies and his team have gathered evidence for this interaction by combining protein crystallography with computational analysis. ‘The big breakthrough for us has been seeing the crystal structures of a number of proteins now where, because of the way the protein crystallised, they leave a solvent channel next to the ice binding site,’ Davies explains. As a result, there are no constraints on the way that water molecules interact with this face of the protein – and the results are quite ice-like, Davies adds.
‘The other method that is becoming quite reliable is molecular simulation,’ he says. ‘Because we have crystal structures, we can actually check our answers, and we find that the latest programs are doing a very good job of predicting where waters will be on a protein surface – it matches what we see in the x-ray crystal structure.’
Davies says that he would like to see this ice-like water in a few more crystal structures to be completely convinced that this mode of ice binding is truly representative of all antifreeze proteins. Antifreeze proteins are, unfortunately, very prone to crystallising with their flat, hydrophobic ice-binding faces pressed together in a protein–protein interaction, squeezing out any water molecules. ‘One of the things we are doing is to bind a bigger protein to the antifreeze protein and getting it to crystallise, in the hope that by chance the ice binding site will be in a freer environment exposed to solvent. We’re seeing some progress on this but it’s still early days,’ he says.
A smoother ice cream
Within the last decade, the first commercial applications for antifreeze proteins have begun to emerge. If you have eaten a Unilever ice cream or ice lolly recently, it may have contained what the company calls an ‘ice-shaping protein’. The idea here is not to lower the ice cream’s freezing point, but to exploit another facet of antifreeze proteins’ ice-binding property – their ability to restrict the size of the ice crystals. By adding a tiny quantity of the protein, the ice cream’s fat content can be cut without compromising its smooth texture. Products incorporating the ice-shaping protein have been sold in countries such as the US and Australia for several years, and in 2009 the company received EU approval to use the ingredient in ice cream sold across all member states.
‘Unilever have done a very good job of making food-grade antifreeze protein, at very low cost,’ says Davies. As the protein would be impossible to harvest from the wild, the company produces it using genetically modified yeasts.

For certain other potential applications, it is consumer concern over eating GM organisms that is holding antifreeze applications back, Davies suspects. ‘One of the original applications that we looked at was freeze-tolerant salmon,’ he says. The idea would be to add antifreeze genes into fish or crops so that they could be grown over a longer season, or in areas currently too cold to grow them. ‘We have enough information to make a salmon with the same degree of protection as native fishes in the Arctic or Antarctic. Whether or not the fish farmers would want to go that way, given the amount of resistance there is to GM organisms, I don’t know,’ he adds.
For other, much-needed applications – cryopreservation of donated organs for transplant, for example – antifreeze proteins have not proven the success that was initially hoped. ‘If you try to do cryopreservation with them, they cause more damage than protection,’ says Matthew Gibson, a polymer chemist working on antifreeze compounds at the University of Warwick, UK.
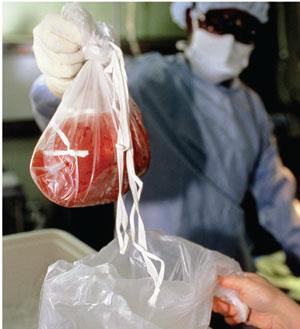
The trouble comes when you try to thaw out the preserved tissue for use. ‘The outside melts before the middle, and at the interface there’s quite a lot of energy,’ explains Gibson. As a result, rapid ice recrystallisation begins, with highly damaging results. Because of the way that bound antifreeze proteins block ice growth on the edges of the ice crystals, they start to grow from the top and bottom instead, into sharp needle-shaped structures. ‘These pierce cell membranes and cause all sorts of problems,’ he says.
Gibson and his team are looking for materials that will inhibit ice crystallisation without lowering the freezing point or creating dangerously shaped crystals. ‘We took the view that the repeat protein looked a bit like a polymer. So our hypothesis was, can we make some synthetic polymers to have all this property, but be scalable,’ he says.
As a starting point, the team examined known polymers for their antifreeze properties – and hit upon a rather unlikely candidate.6 ‘We found that polyvinyl alcohol [PVA] – wood glue – is one of the best recrystalisation inhibitors anyone has ever found,’ says Gibson. ‘Price-wise you’re onto a winner, and in several countries it is approved for food usage,’ he adds.
Understanding quite why PVA displays these properties is a current topic of research in Gibson’s lab. ‘It doesn’t have the precise rigid tertiary structure of proteins, and we don’t really know why it works. As the polymer gets longer it seems to be better, and we’re not sure why that’s the case,’ he says.
The suggestion that size matters for PVA’s recrystallisation inhibition effects is in contrast with the work of Robert Ben at the University of Ottawa in Canada, who uses a synthetic organic chemistry approach to develop synthetic analogues of antifreeze proteins. Ben has shown that a small molecule surfactant called octyl galactopyranoside, and a hydrogelator called octyl-gluconamide, are potent ice recrystallisation inhibitors.7
Ben’s results suggest that his recrystallisation inhibitors work differently to natural antifreeze proteins. They do not seem to bind to the ice at all, apparently preventing ice crystal growth by sitting at and disrupting the boundary between the quasi-liquid layer of water around an ice crystal and the bulk water.
‘Maybe there isn’t one mechanism,’ says Gibson. ‘Maybe there are lots of molecular features that can lead to these properties, and just because ice-binding proteins do it one way, does that mean it’s the best?’
James Mitchell Crow is a science writer based in Melbourne, Australia
References
1 A L DeVries and D E Wohlschlag, Science, 1969, 163, 1073 (DOI: 10.1126/science.163.3871.1073)
2 J G Duman and A L DeVries, Nature, 1974, 247, 237 (DOI: 10.1038/247237a0)
3 A L DeVries, Science, 1971, 172, 1152 (DOI: 10.1126/science.172.3988.1152)
4 C P Garnham, R L Campbell and P L Davies, Proc. Natl. Acad. Sci USA, 2011, 108, 7363 (DOI: 10.1073/pnas.1100429108)
6 M I Gibson, Polym. Chem., 2010, 1, 1141 (DOI: 10.1039/C0PY00089B)
7 C J Capicciotti et al, Chem. Sci., 2012, 3, 1408 (DOI: 10.1039/C2SC00885H)
8 K R Walters et al, Proc. Natl Acad. Sci. USA, 2009, 106, 20210 (DOI: 10.1073/pnas.0909872106)









No comments yet