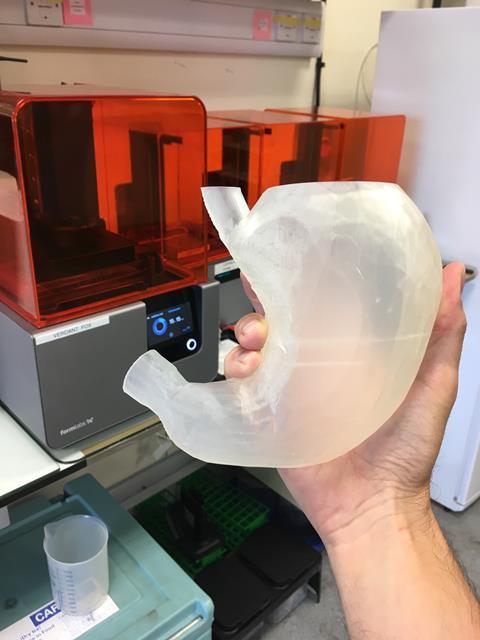
In April 2014, the Mata twins were born into an uncertain future. The sisters were connected from their chest to their pelvis with several shared organs, and it was deemed desirable to separate them. Faced with one the most complex separations ever for conjoined twins, their surgeons at Texas Children’s Hospital in Houston, US, embraced the help of an unexpected rookie: a 3D printer.
The girls first underwent a series of computerised tomography scans. This data was used to print out colour-coded 3D models of the girls’ organs and skeletons in exquisite detail. These models enabled the separation surgery to be planned and extensively practiced. At 10 months old, the girls were separated 18 hours into a 26-hour operation. ‘This is the first time a separation surgery for twins with this particular configuration has been successful,’ said one of their surgeons at the time. Along with the surgeons, 3D printing was hailed a hero.
And it isn’t just for conjoined twin separation that 3D printing has earned its stripes in the operating room in recent years. Other types of complex surgeries have now been practiced this way, including a full-face transplant, and spine, brain and heart surgery. 3D printing is also increasingly being used to produce low-cost, made-to-measure implants including jaws, pieces of skulls, and hips.
To date though, healthcare’s most prolific use of 3D printing is in the production of hearing aids. A silicone impression of a wearer’s ear is scanned and the data used to print out personalised digital aids that fit in the ear, or soft earmoulds for behind-the-ear devices. A once labour-intensive process has been automated and produces snug earpieces that are both comfortable and prevent sound leakage.
Drug production
3D printing is also starting to make a name for itself in medicine manufacturing. While it’s hard to foresee the wholesale replacement of current tablet manufacturing processes, 3D printing is expected to find a place in certain niche medications and in personalised tablets.
In 2015, the US FDA (Food and Drug Administration) approved the first – and currently only – 3D printed tablet. Spritam is a reformulation of the anti-epileptic seizure drug levetiracetam developed by Aprecia Pharmaceuticals, based in Cincinnati, US. Spritam has a layered, highly porous structure that rapidly sucks in liquid, collapsing to form a suspension. Its unique structure can’t be achieved using traditional manufacturing methods.

‘Using 3D printing we create this latticework which, when it touches a sip of water, disperses in the mouth within seconds,’ explains Tim Tracy, Aprecia’s chief executive. This can be useful for children, the elderly and other patients who may struggle to swallow pills. It also allows very large doses of levetiracetam to be included in a single, small tablet. ‘We can put upwards of a gram of API [active pharmaceutical ingredient] into a dosage form and the dispersion in the mouth is virtually identical regardless of the strengths,’ Tracy explains.
Aprecia uses a proprietary powder bed and inkjet 3D printing technology known as ZipDose. First, a powder layer that contains the drug is laid down along something much like a conveyor belt. It then passes under an inkjet printhead that prints a binding liquid at specific locations along the powdered sheet, binding the powdered layer together at those spots. ‘We then lay down a second layer of powder and print again in exactly the same places,’ says Tracy. ‘We print layer after layer after layer, upwards of 30, even 40, times, depending on the size of the tablet that we’re trying to make.’
‘We can print tens of thousands of tablets a day on a single printer,’ explains Tracy. This contrasts the prevailing perception that printing in 3D is always going to be slow. Aprecia has developed 3D printing for mass production, using printers that are ‘the size of a room’ rather than the more familiar desktop size.
3D printing drugs has potential beyond high-dose tablets that can instantly dissolve. In December 2017, Aprecia and Cycle Pharmaceuticals from Cambridge in the UK announced a partnership to develop and commercialise 3D printed tablets for orphan (rare) diseases using this ZipDose technology.
For so-called orphan drugs, the inherent versatility of 3D printing is particularly appealing. Rather than the current situation of pharmaceutical companies needing to maintain expensive specialist infrastructure to manufacture medicines of which low numbers are sold, it is theoretically possible to print many different types of tablets by simply changing the powder used in Aprecia’s ZipDose process, or even by just changing the ‘ink cartridges’ in commercially available 3D printers.
We can print tens of thousands of tablets a day on a single printer
Tim Tracy, Aprecia Pharmaceuticals
This changing-the-ink-cartridge concept also offers the possibility of decentralising tablet manufacture in certain circumstances. ‘You could imagine a situation where a printer is making medicines much more locally to patients, perhaps in a hospital or even a pharmacy,’ explains Clive Roberts from the school of pharmacy at the University of Nottingham in the UK.
It also opens up the possibility of using 3D printing in personalised medicine – to produce a tablet designed to meet the needs of a single patient. ‘You can change the drug loading, the combination of drugs and the release profile of the formulation to bespoke for an individual patient, in other words personalisation, at no extra cost,’ says Roberts.
Spritam’s approval is widely viewed as the start of a 3D printing revolution in medicine manufacturing. ‘More than a dozen pharmaceutical manufacturers have formally or informally been in contact with the FDA’s Center for Drug Evaluation and Research regarding the use of 3D printing to manufacture drugs,’ FDA spokesperson Jeremy Kahn told Chemistry World.
Many pills in one
Roberts’ lab has been using 3D printers to produce polypills. The idea of one tablet containing multiple drugs has been around for a while, as a means to reduce the number of different tablets a patient with complex medical needs must remember to take. But drug compatibility concerns and issues with drugs often needing to be released on different timescales once inside the digestive system has hindered their development. 3D printing navigates these problems by allowing multiple drugs to be compartmentalised within a single tablet. In 2015, Roberts showcased two polypills: one containing three drugs, which were all released at different rates, and a second containing five drugs, two compartmentalised together for immediate release and three in separate compartments for sustained release. No interactions between the different drugs were observed in either polypill.
More recently, Roberts’ lab has been exploring how changing the geometry of a 3D printed tablet changes the speed at which the drugs contained within it are released. ‘3D printing allows you to change the distribution of a drug in a single tablet. You can put a large concentration of drug in the centre, decrease the concentration as a gradient going out and that then modifies the release profile,’ he explains. ‘3D printing gives you an opportunity to think about things that you would not have been able to achieve using standard manufacturing.’
3D printing allows you to change the distribution of a drug in a single tablet
Clive Roberts, University of Nottingham
For this and their polypill work, Roberts’ lab use commercially available 3D printers with the APIs (and other components that make up the medicines) contained within either an ink for inkjet printing or a paste for extrusion. ‘With paste extrusion 3D printing, imagine you are icing a cake and you’re squeezing out a paste which then rapidly forms a solid once printed,’ he says. This concept sounds easier than it is in practice, and the majority of his lab’s research effort is invested in developing pastes and ink formulations that allow the necessary precise control over the structure of the printed tablets.
High street printers
The type of 3D printer that most people are familiar with is FDM (fused deposition modelling ), where a thermoplastic wire on a spool is fed into a printer, melts and is printed out in a semi-molten state. Once printed, the plastic rapidly re-solidifies. These printers are used by hobbyists to make cookie cutters, door stoppers and the like. They are also increasingly finding a home in laboratories to make small, simple plastic items such as Keck clips and funnels cheaply from publicly shared designs. Chemists are also starting to branch out into more complex plastic, ceramic or metal designs (see Chemistry World, March 2018, p34). The use of FDM 3D printers for medicine manufacturing is being explored by some researchers including Stephen Hilton at University College London’s school of pharmacy in the UK.

‘There are already organic compounds – the colours – in thermoplastics,’ he explains. ‘Medicine printing is similar in that the coloured compound can be replaced with a drug to make a drug-containing plastic.’ He embeds the drug into a plastic using a process called hot-melt extrusion (an established technology for mixing drug molecules with polymers under mechanical pressure). The resulting filament is loaded into an FDM print head and printed. Hilton’s team is currently looking at how to make this filament less brittle.
There are challenging but inherent issues with using FDM printers for drug formulations, including their slow speed and issues with the thermal degradation of the drugs being printed. ‘We tend to print at about 100ºC,’ explains Hilton. ‘Some anticancer drugs do very well, whereas antibiotics tend to degrade very rapidly at this temperature.’ He adds that for thermally unstable drugs, and more rapid production, another type of high street 3D printer may be more appropriate: stereolithography, or SLA. This method also offers a higher resolution print in all dimensions than their high street FDM counterparts. The Hilton lab is also exploring the potential of this type of printer for printing tablets.
For SLA printing, the drug is placed in a liquid resin composed of acrylate-based monomers and a photo initiator. The resin is poured into a clear shallow tray and loaded into the printer. Under the tray sits a laser and a mirror. When the laser hits the resin it triggers polymerisation to a solid polymer at that exact spot only. ‘The laser traces a path in the liquid and prints up a [solid tablet] layer by layer,’ says Hilton. As the print progresses, the solid tablet raises out of the liquid resin tray. Once completed, the excess resin is drained away for reuse. The tablet is then placed under UV lamps to polymerise any remaining monomers. Toxicity is a general issue with acrylate-based resins, explains Hilton. ‘We have to modify them quite heavily to make them biocompatible.’ Again, resin development is a large part of Hilton’s work.
Like Roberts, Hilton is also looking at 3D printing sustained-release drugs but is taking a different approach. He is designing tablets that physically change shape, rapidly expanding when they come into contact with acid in the stomach. ‘When you swallow the tablet, it passes down into the stomach, the outer capsule dissolves, releasing the drug device inside which then grows to roughly the size of a ping pong ball [40mm],’ he says. The tablet is then retained in the stomach because it is too large to pass through. ‘It’s an open structure that slowly breaks down and releases a drug over a seven day period.’
‘For our tablets, 3D printing is necessary; they cannot be made with conventional manufacturing methods,’ he adds. The resins used for this purpose were designed in Hilton’s lab. ‘The device twists and compresses for packing into the tablet, and then opens up into a much larger structure,’ he explains. ‘It’s a biocompatible material that is also flexible and effectively has shape memory.’ The concept of printing 3D objects which then reshape themselves over time is known as 4D printing.
3D bioprinting
3D printing is also making headway in the pharmaceutical industry as a way to produce 3D groups of cells for use in high throughput screening. Compared to typical 2D cell assays, 3D groups offer a more realistic cellular environment. This potentially enables efficacious compounds to be identified more easily, but also allows game-ending toxicity issues to be identified much earlier in the drug development process.
Conceivably, 3D bioprinting could speed up drug discovery. ‘It might allow us to get to the chase much quicker,’ explains Prasad Shastri from the institute for macromolecular chemistry at the University of Freiburg in Germany. ‘The pharma industry is already very much engaged in trying to bring 3D bioprinting into their discovery process,’ he says.
As with printing drug formulations, there are a number of different types of 3D bioprinters and Shastri’s lab focuses on the extrusion-based printers. His interest lies in the development of the paste, the bioink. ‘The off-the-shelf products are very subpar,’ he says, giving poor spatial control over the printed cell groups. If you can’t consistently print the same 3D cell group each time, the reproducibility of screening results obtained using them is an issue, Shastri explains. ‘The key thing for a field like this to succeed is that the information that people are able to generate is reproducible.’
The development of bioink has an extra complication compared to standard ink for 3D printing; the need to disperse the cells a hydrated environment. ‘Hydrogels are the only material that meet this key criteria,’ he says. ‘[Bioink development] is one space where there’s going to be a lot of innovation in the next few years. If this innovation doesn’t occur, 3D bioprinting will stagnate.’
If you believe the hype, 3D printing is destined to transform every aspect of our lives at home, in hospitals and in our labs. Industrial and academic scientists are currently scrambling to develop viable tools of the trade to enable its continued charge into the pharmaceutical arena, but only time will tell whether 3D printing will live up its disruptive technology potential here.
Nina Notman is a science writer based near Salisbury, UK






















No comments yet