The challenge of finding drugs for a poorly understood disease with many symptoms is clear. Clare Sansom looks at the work going on to help the people suffering in its shadow
Covid-19 was first designated a ‘public health emergency of international concern’ by the World Health Organization (WHO) on 30 January 2020. That designation was finally lifted on 5 May 2023, long after the last lockdown had ended. Pandemics, however, do not answer to international decrees. As the security advisor Brian Michael Jenkins wrote in Time magazine, pandemics throughout history have always had ‘ragged endings’, and a new variant of the virus is already generating an uptick in cases. Nevertheless, the consensus seems to be that the world should move on from the pandemic to other pressing concerns.
Not everyone agrees, however. Among the dissenters will be the estimated 65 million people worldwide who have at one time received a diagnosis of ‘post-acute Covid syndrome’, more commonly known as long Covid. This is an astonishing number; given that the figure of around 700 million Sars-CoV-2 infections currently quoted by the WHO includes many reinfections, it represents well over 10% of people ever diagnosed with that infection. In less than four years since the virus emerged, a population equivalent to that of France have had their quality of life diminished or their lives completely put on hold by lingering symptoms, often severe, linked to an original infection. Most patients do seem to improve over time, but their recovery is painfully slow. An observational study recently published in the journal Lancet Regional Health – Europe found very few complete recoveries in a population of over 300 long Covid patients over two years. The authors’ conclusion that this illness ‘[poses] a major challenge to healthcare systems’ is hardly surprising.
The WHO’s formal diagnostic definition of long Covid is ‘… the continuation or development of new symptoms 3 months after the initial Sars-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation’. That encompasses a multitude of problems. Whereas there are a handful of very common ‘classic’ symptoms - fatigue, breathlessness, loss of smell, muscle pain and the neurological condition usually described as ‘brain fog’ – the complete list includes about 200 possible ones. Long Covid can occur after an original infection that was trivial or even symptomless, but anecdotally at least it is more severe and longer lasting when the original infection was itself severe.
A mix of symptoms
The fatigue that is probably the commonest and best-known symptom of long Covid is often trivialised by people who haven’t experienced it, simply because ‘we’ve all been tired’. But fatigue as experienced by long Covid patients is not like that associated with pulling all-nighters or even running marathons. People with long Covid cannot recover from fatigue by simply resting, and often wake up feeling tired. The clinical description, ‘post-exertional malaise’ (PEM), is more accurate. It describes a devastating condition in which even gentle activity can precipitate a ‘crash’ that can make the patient bed-bound for days or weeks. ‘There are no drugs to treat PEM, and the only treatment that works is “pacing” your activities to keep below the level that will precipitate a crash,’ says Julia Moore-Vogel, a computational biologist by training and long Covid researcher at Scripps Research Translational Institute in La Jolla, US, who has herself suffered from long Covid since 2020. ‘And you will still end up doing perhaps 10% or less of what you need or want to do… with PEM, you feel as if your internal battery is constantly drained.’ She uses a Garmin smartwatch to help with pacing.
We don’t have reliable biomarkers for ME/CFS and long Covid-19 the way we do in many other conditions
Post-exertional malaise is one characteristic symptom of another poorly understood and often maligned syndrome, known variously as myalgic encephalomyelitis, chronic fatigue syndrome or by its initials as ME/CFS. Like long Covid, this is a heterogeneous condition, with many possible symptoms alongside the fatigue, and like long Covid, patients will fluctuate between good and bad days. There is still a great deal of controversy over its origin, but very many cases seem to have been triggered by an initial infection. It is possible, even likely, that others were triggered by an initial infection that was too trivial to be recognised.
Vogel manages The Participant Center, part of the All of Us Research Program, a National Institutes of Health funded study. This large population health study is recruiting up to a million volunteers to represent the diversity of the US population. These volunteers share electronic health records, submit measurements and blood and urine samples, and complete health surveys; this data forms a resource that can be used for large-scale studies of many diseases, including ME/CFS, Covid-19 infection and its aftereffects. ‘We don’t have reliable biomarkers for ME/CFS and long Covid-19 the way we do in many other conditions,’ she adds. ‘But we can measure patients’ progress – or the lack of it – using questionnaires and devices like the Garmin.’
Analysis of data from long Covid patients submitted to All of Us shows that they can be clustered into groups depending on their most prevalent symptoms. One group of patients have frequent gastro-intestinal symptoms; another group suffers from postural tachycardia syndrome, in which standing causes the heart rate to increase, leading to faintness; others’ symptoms are dominated by fatigue. In such a complex condition, it is particularly difficult to drill down to the molecular level find the links between the original infection and subsequent pathology, and thus the root cause of the disease. This is important (although perhaps not strictly necessary) if we are to discover drugs that will modify the course of the disease rather than just treating its symptoms.
Untangling the causes
Akiko Iwasaki, an immunologist working at Yale School of Medicine in Connecticut, US, highlights four distinct hypotheses for the root cause of long Covid: persistence of infectious virus or of viral RNA and proteins in a patient’s body; an autoimmune response triggered by the original Sars-CoV-2 infection; reactivation of latent viruses such as the Epstein-Barr virus; and organ and tissue damage triggered by inflammation. She sees them as interlocking ‘wheels within wheels’. ‘These hypotheses are not mutually exclusive, but they will affect different patients in different proportions, and the differing molecular pathologies may give rise to distinct clusters of symptoms,’ she says.
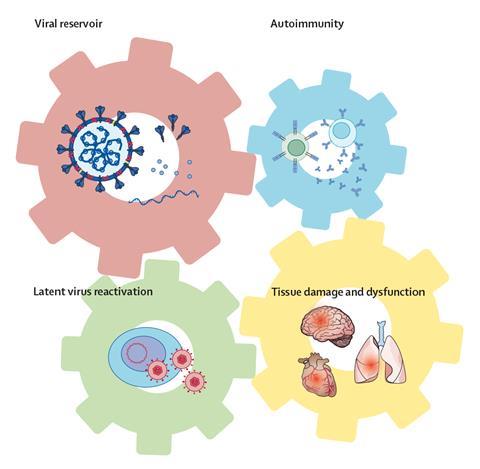
The drugs that are most suited to each subtype might differ, too. Underlying Sars-CoV-2 infection could be treated with the same antivirals that are used for the acute disease; drugs that block cytokines and cytokine signalling could dampen an autoimmune response; a latent infection could be targeted with the relevant antivirals; and organ and tissue damage could be treated symptomatically. ‘We are looking at biomarkers related to immune function and dysfunction, in the hope of identifying patients who are more likely to respond to one or other of these therapies,’ Iwasaki adds.
Iwasaki and her team are investigating the first of these hypotheses – persistent viral infection – through a clinical trial of an established antiviral, Paxlovid, in long Covid patients. This drug was one of the first to show significant efficacy in acute Covid-19 infection. It is a combination of two protease inhibitors, nirmatrelvir and ritonavir. Nirmatrelvir, which was developed specifically to target the Sars-CoV-2 virus, prevents that virus’ main protease from cleaving the polypeptide encoded by the viral genome into separate proteins and thus forming mature daughter virions. Ritonavir was developed as an inhibitor of HIV’s protease, and it is still prescribed as part of the highly active anti-retroviral treatment (HAART) combinations that have so improved the prospects for people with HIV infection since the 1990s. However, its main role in the Paxlovid combination – and now, in HAART – is not as an antiviral but as an inhibitor of the cytochrome P450 isoform CYP3A. This enzyme breaks down nirmatrelvir leading to its excretion in urine, so combining that drug with ritonavir increases its concentration in the bloodstream and thus its efficacy.
‘We guess that only some of the patients recruited into the study will have persistent virus, so we will need to recruit a lot of patients to see a positive signal in the results,’ says Iwasaki. ‘It would be much easier if there was an easily measurable biomarker for persistent infection, so we could select exactly those patients we thought would be most likely to benefit.’
Iwasaki and her colleagues were able to move straight on to a placebo-controlled phase 2 trial with Paxlovid because this combination therapy is already on the market. This is an example of drug repurposing, in which a drug used in one disease is tested in, and may be prescribed for, another (or, as in this case, a different form of the same disease). This principle forms the basis of the large, long-standing Recovery trial for severe acute Covid infection that has so far identified four widely used drugs, including the cheap, simple steroid dexamethasone. This process is obviously far faster and cheaper than designing a drug from scratch, and it has other advantages: the pharmacodynamic and pharmacokinetic profiles of the drugs are known (hence there was no need for a Phase I trial to demonstrate the safety of Paxlovid), and there will generally be efficient routes to their synthesis.
A question of genetics
Another way of stratifying long Covid patients is to look at the genetic risk factors for developing the disease. After all, approximately 90% of all people who contract Sars-CoV-2 infection do not develop a long term post-viral condition: it is worth asking what, genetically, can distinguish them from the 10% who do, and also whether there are genetic differences between long Covid populations with different clusters of symptoms.
Scientists at PrecisionLife, a computational biology company based in Oxford, UK, have developed a method of drilling down into large genetic and phenotypic datasets of patients with complex diseases, identifying genetic differences that distinguish between sick and healthy individuals and also between patients with different forms of the same disease. This enables the identification of diagnostic biomarkers for disease subtypes, ‘druggable’ protein targets, and, often, existing drugs that might be repurposed. So far, the method has been used to analyse over 50 chronic diseases including ME/CFS and both acute and long Covid, identifying over 300 potential protein targets and over 250 current drugs that might be repurposed.
What we are developing now are biomarkers to stratify each patient’s disease accurately at the molecular level
Some of these seem, on the surface, to be unlikely candidates for their new indications. ‘One of the potential treatments we identified for acute Covid-19 infection is dutasteride,’ says Steve Gardner, chief executive of PrecisionLife. ‘This drug is commonly used to treat benign prostate enlargement, but genetic profiling has suggested a molecular mechanism through which it might help about a third of all acute Covid-19 patients.’ This drug is now in clinical trials.
Turning to long Covid, Gardner and his colleagues used their combinatorial approach to analyse genomic and health data from about 2000 long Covid patients divided into two subsets: those with a severe, multi-system form of the disease and those with a fatigue-dominant condition resembling ‘classic’ ME/CFS. This analysis identified a total of 73 genes with variants or expression changes that were more common in either or both long Covid cohorts. Forty-two of these genes encode proteins that are considered ‘druggable’: 26 by antibodies and 18 by more tractable small molecules. These include TLR4 (toll-like receptor 4), which encodes a transmembrane protein involved in the innate immune system response; the aptly named CLOCK gene, which encodes a protein involved in controlling the circadian rhythm and is associated with the fatigue-dominant subset only; and genes involved in mitochondrial function and insulin signalling. Thirteen known drugs that target these proteins are now in clinical trials for long Covid, and some are also being investigated as potential targets for acute Covid-19 infection or for ME/CFS. ‘What we are developing now are biomarkers to stratify each patient’s disease accurately at the molecular level, and, just as importantly, to predict which acute Covid-19 patients are most likely to develop the long form,’ adds Gardner.
Finding treatments
The inclusion of insulin signalling in a list of functions affected by long Covid is, on reflection, not particularly surprising because of links that clinicians have observed between Covid-19 and diabetes. Interestingly, the relationship between the two diseases is ‘bidirectional’; patients with diabetes are more likely to be severely hit by Sars-CoV-2 infection, and some Covid-19 patients develop diabetes soon after recovery. This is frequent enough for some experts to consider diabetes to be a ‘post-infection sequel’, or even a special form of long Covid. Both type 1 and type 2 diabetes have been recorded in newly recovered Covid-19 patients, and a registry called CoviDIAB has been set up at King’s College London in the UK to hold details of all new cases of diabetes that have been linked to Sars-CoV-2 infection.
Metformin reduces the risk of long Covid by about half
In the long term, however, the most important link between long Covid and diabetes may come through the repurposing of a well-known diabetes drug. Early in the pandemic, clinicians observed that, although diabetes in general pre-disposed patients to severe Covid, those who were already taking metformin for type 2 disease did rather better. This is a small, simple molecule, first discovered in 1922, and the exact mechanism through which it reduces glucose levels in type 2 diabetes patients is still not wholly understood. It has, however, been considered as a possible drug for several other diseases, including viral ones, and cheminformatics analysis identified it as a possible treatment for acute Covid-19. It was tested alongside two other drugs, fluvoxamine and ivermectin, in the complex Covid-OUT clinical trial for acute Covid infection but the primary outcome was not defined well since the definition of severe Covid-19 changed as more was learned about the disease..
A long-term outcome in that trial, however, proved much more noticeable. ‘We gave patients metformin or placebo for two weeks towards the start of their initial infection, and by day 300 – that is, about ten months in – the risk of developing long Covid was about 40% lower in the metformin group, and 60% lower if metformin was started early in the infection,’ explains study leader Carolyn Bramante from the University of Minnesota Medical School in the US. ‘This is very significant for prevention: metformin is a generic drug that is cheap to make and safe to use even in pregnant women and children, and these results – with the risk reduced by about half – mean we can already think about how to roll it out.’ Clinicians will need to consider which Covid-19 patients should receive metformin, and when; again, validated biomarkers – in this case, for long Covid risk – may help answer these questions.
Long Covid and ME/CFS are just two of a whole cluster of chronic conditions triggered by an initial, and often trivial, infection. Similar ‘post-acute’ syndromes can arise after infection by many different pathogens, including parasites and bacteria. The latter category includes Coxiella burnetii, which can cause a long form of Q fever, and the tick-borne Borrelia bacteria that cause Lyme disease: both these have chronic fatigue or PEM as a presenting symptom.
Lyme disease is a heterogeneous condition that shares many of its constellation of symptoms with long Covid, and it is believed to arise from one of Iwasaki’s ‘root causes’: lingering traces of the infectious agent. Broad spectrum antibiotics are the main therapy, but these are not always successful, particularly if treatment is delayed. No Borrelia-specific antibiotics are yet in the clinic, but there is at least one in development. Hygromycin A, a modified cinnamic acid discovered in the 1950s but long ignored, was recently found to selectively kill these bacteria and clear a Lyme-like disease from mice. Unfortunately for many thousands of Lyme patients, however, more research is still needed before it can enter clinical trials.
Long Covid was described in a recent editorial in the Lancet Respiratory Medicine as ‘a growing public health crisis on a global scale that demands a focused, well-resourced and patient-centred response’. With the Sars-CoV-2 virus still circulating and mutating, it seems likely that new long Covid diagnoses will continue to outnumber cures for some time. But successful clinical trials are showing light at the end of the tunnel, and medicinal chemists are among those leading the way. And the more we can learn about long Covid, the more we should be able to offer patients with ME/CFS, Lyme disease and other long-haul syndromes.
Clare Sansom is a science writer based in Cambridge, UK
















No comments yet