The next generation of battery technologies might pack significantly more power into the electric cars and mobile devices of the future. James Mitchell Crow reports
It is not difficult to think of a better design for a battery than today’s lithium-ion rechargeables, says Xiaodan Huang. ‘There are many, many concepts for new battery designs that can provide better energy density, better power density, give faster charging properties, than current lithium-ion batteries,’ says the battery materials researcher from the University of Queensland in Australia. ‘But when we try to bring these batteries from theory to industry, from fundamental knowledge to prototype – that’s difficult,’ Huang adds.
Some promising battery concepts have been stuck at the research phase for half a century, but they may not languish in labs for much longer. Sparked by the nascent multi-sector electrification boom, an intense effort is now underway to bring high-performance battery technologies into real world use. One hotspot area of research is on batteries suited to grid scale electrical energy storage (see The long-term storage problem). Another driver is the demand for electric cars.
Lithium is the smallest, lightest metal – and eager to give up an electron
‘If you just look at the laws passed in the US and Europe around electric vehicles and emissions, and you do the sums on the size of the market, there is complete certainty that these new batteries are coming,’ says Matthew Hill, a battery chemist at Monash University in Melbourne, Australia. How quickly these next-generation battery concepts finally emerge from the lab is the big question, he adds. ‘If we nickel and dime along, there’s no known end to this situation – but if we put in a serious investment, then in a couple of years’ time we’ll start to see products coming.’
Pedal to the metal
To make a better battery for mobile applications such as phones, laptops or electric vehicles, the defining design parameter is to pack the maximum electrical energy into the smallest, lightest package possible. If you were to go back to the periodic table, and design such a battery from scratch, it is still hard to go past lithium (see Lithium short box below). It’s the smallest, lightest metal – and eager to give up an electron.
‘In terms of whether there are brand new chemistries that can have the energy density competitive with lithium, at this moment the short answer is no,’ argues Ping Liu, a battery researcher at the University of California, San Diego, US. But that’s not to say we couldn’t make lithium-based batteries with many times the energy density of today’s commercial batteries, he adds.
Rather than lose the lithium, many beyond-lithium-ion battery concepts aim to eliminate some of the baggage weighing the current generation technology down. Starting with the anode.
Commercial lithium-ion batteries invariably use graphite as an intercalation anode. The lithium ions nestle between individual layers of the graphite host. ‘With graphite you build a happy house for lithium,’ Liu says. But the graphite adds bulk and weight to the battery, lowering its energy density.
Lithium short
Lithium may remain the metal of choice for mobile applications, but such is the predicted need for batteries as the electricity grid and transportation sectors decarbonise, lithium demand could outstrip supply.
Aluminium could transfer three electrons for each atom
Numerous alternative battery chemistries, employing different metals, are in development. Many, however, are bulkier and heavier than lithium batteries, better suited to fixed grid- or household-level storage rather than mobile applications. ‘There is a lot of enthusiasm about the sodium-ion battery, for example, but that is a low-energy-density battery suitable for stationary storage,’ says Ping Liu, a battery researcher at the University of California, San Diego, US.
One possible exception to battery bloat could be aluminium, which counters its extra weight over lithium by carrying more charge. ‘Aluminium can ideally transfer three electrons for each atom, which theoretically gives it more capacity to store electricity,’ says Xiaodan Huang, a University of Queensland battery materials researcher.
Aluminium is also much more abundant, and cheaper, than lithium. Plus, it is safer, Huang adds. ‘Unlike lithium they are not flammable to air or water,’ he says.
In 2015, Hongjie Dai group at Stanford University in the US showed that – akin to today’s lithium-ion batteries – a graphite electrode might offer a practical way to get aluminium batteries up and running. The basic architecture of a typical aluminium battery consists of an aluminium metal anode, and a graphite cathode to store aluminium ions between its layered carbon structure.
Graphite’s quite rigid structure and narrow space between layers limits aluminium storage
But the aluminium ion’s triple charge is a significant challenge when it comes to the practicalities of battery performance, notes Liu. In the typical chloride-based electrolytes used in aluminium batteries, aluminium will not exist as the simple Al3+ ion, he says. ‘The ion is too polarising, it will acquire anions and make a complex, like AlCl4–. You are essentially running a battery based on those ions rather than aluminium itself.’ This complexation causes problems from the mass balance point of view of ion transport, as well as complications in interactions with the cathode.
Huang has been exploring more accommodating carbon-based cathodes. ‘Graphite’s quite rigid structure, and relatively narrow space between layers, limits aluminium storage,’ he says. ‘We use graphene nanosheets that we make holes in, to allow more ions in.’ Huang mixes the graphene sheets with a polymer that, when heated, breaks down to generate free radicals that cut holes in the graphene sheets.
The team is now working on a scaled-up prototype, although increasing the size means confronting issues of mass transfer. ‘But this year we have a breakthrough, a method to press the graphene materials to increase their density and reduce the thickness when they are loaded in the battery.’ The compressed, holed material shortens the ion transfer pathway. ‘We saved 80% of the volume, which significantly reduced the transport pathway length,’ Huang says. ‘Hopefully this will lead us to a mature prototype.
‘We think aluminium batteries have the potential to, if not replace lithium, at least provide a competitive technology to lithium batteries in the market,’ Huang adds.
Alternative models
One route to a higher capacity next-generation battery could be to replace graphite with an alternative anode host material. Graphite has a charge capacity of 372mAh per gram, but silicon, which can soak up lithium ions via an alloying reaction at the anode, has a capacity of up to 2009mAh per gram. Charging the battery causes the silicon to swell up by 400%, however, a volume change 40 times greater than graphite’s. This extreme expansion and contraction fractures the silicon anode, leading to poor battery cyclability.
Issues associated with silicon have led many researchers to sidestep it and look to the ultimate lithium battery anode material: lithium metal itself. Made of the active charge carrying material, a lithium metal anode has a towering 3860mAh per gram theoretical capacity.
Stanley Whittingham’s original work to develop the lithium battery in the early 1970s – for which he won the 2019 chemistry Nobel – used a lithium metal anode. But so far, a practical lithium metal battery has proven elusive.
Charging and discharging a lithium metal battery is fundamentally an electrochemical lithium plating and stripping process. But it’s imperfect. ‘Just like any electroplating, you can’t guarantee to plate a perfectly smooth lithium structure,’ Liu says. ‘And when you strip the lithium, it doesn’t dissolve uniformly either.’ Within a few recharge cycles, a block of lithium can start to resemble a sponge. ‘Lithium is very reactive in electrolyte, so the higher the surface area, the higher the reactivity, and you start to lose lithium.’
Whittingham’s lithium battery concept only became commercial reality once this problem was avoided with the introduction of carbon-based anodes. ‘When we move from graphite to lithium, we invite a lot of really difficult challenges back,’ Liu says.
Lithium fluoride has been a magical compound
One area of research has focused on modifying the electrolyte so that it binds the lithium less tightly and allows it to plate out more easily. Another direction has been to use external force, such as pressure, to heal the holey electrode. ‘Lithium is very soft, so even if you don’t plate very well sometimes you can just squeeze it into shape,’ Liu says.
In Liu’s lab, his recent work has focused on the surface that the lithium plates onto. Lithium plating involves two interfaces: the anode surface and the electrolyte. Lithium’s high reactivity means the anode is always coated with a passivation layer called the solid electrolyte interface (SEI), produced by electrolyte decomposition (see box Eyeing solutions below).
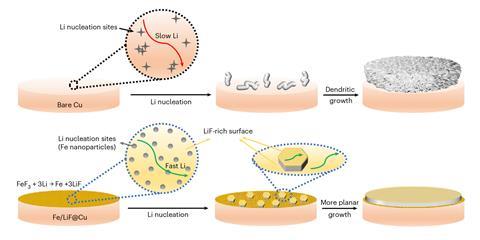
For efficient battery charging, an SEI rich in lithium fluoride has been shown to be beneficial. ‘That has been a magical compound,’ Liu says. ‘People say it is because lithium has very weak interaction with lithium fluoride, so the passivation layer doesn’t slow it down.’ Rapid movement of the lithium atom allows the formation of a smooth, flat anode surface, rather than one covered in branching dendrite spikes.
But according to conventional wisdom, the surface onto which the lithium is plating should be highly lithophilic, so that the lithium likes to stick. ‘Somehow we have imposed a different requirement on the two interfaces involved in lithium plating,’ Liu says. The lithium should move fast through the repellent lithium-fluoride-rich SEI, but then meet a substrate it simply wants to stick to.
Having the incoming lithium ions able to skate about on the surface might be a better route to a smooth, flat lithium surface, Liu suspected. ‘If lithium fluoride is such a magic compound, why don’t we also have it on the substrate side?’ he wondered. His team created a lithium fluoride surface studded with conductive iron fluoride islands as lithium nucleation sites. ‘Once lithium nucleates on iron, its surface diffusion is very rapid and we got these beautiful crystals. So the whole idea that lithium fluoride is magical is true.’ The team now aims to incorporate the concept into a practical battery.
Eyeing solutions
Despite decades of intensive research, certain fundamental questions remain about next-generation lithium battery technologies, hampering their adoption. But new characterisation techniques are increasingly offering new ways to get to grips with these issues.
‘You could argue that the progress we have made with the lithium metal battery is largely due to the use of powerful new tools,’ says Ping Liu, a battery researcher at the University of California, San Diego, US. ‘Using synchrotron x-ray diffraction, we can put the whole battery in and still see through because the beam is so powerful. We can do in operando experiments: while we are charging/discharging we are observing in real time what is happening. These techniques are critical for us to make progress.’
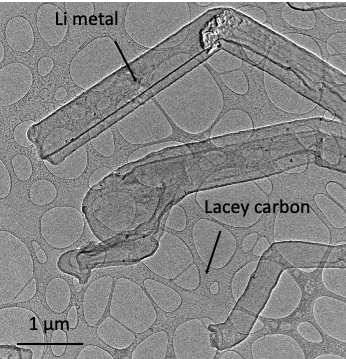
But perhaps the biggest step forward has been the adoption of cryo-electron microscopy (cryoEM) to battery materials research, Liu says. One fundamental question cryoEM is being used to address is the nature of the solid electrolyte interface (SEI) passivation layer that covers the lithium metal anode surface due to a reaction between lithium and the electrolyte.
SEI formation, and the associated lithium loss, is a key issue for battery longevity, says Yuzhuang Li, a researcher at University of California, Los Angeles, US. ‘Right now, the state of the art in lithium metal research labs and start-ups, we can get around 99.5% efficiency, which means we get back 99.5% of the lithium we store each recharge cycle’ says Li. But by losing 0.5% of the lithium to the SEI each cycle, the lithium losses soon mount.
‘If we can understand the fundamental mechanism of SEI formation, and how lithium metal grows in its absence, we can maybe get to 99.9%,’ Li says. In 2017, Li was one of the team that adopted cryoEM – which had proven so powerful in structural biology – for battery analysis. The researchers showed that they could electrochemically deposit lithium battery materials onto the conductive copper ‘grid’ used in cryoEM analysis, while keeping everything under an inert atmosphere.
Using the technique, the team was able to see the SEI structure for the first time. ‘It allows us to look at the interface, and determine the structure of the SEI film, to lend us an understanding of how lithium ions are transported through the layer and lithium metal is growing,’ Li says. ‘Our understanding can potentially help us to minimise SEI formation.’
Thinking positive
In the commercial lithium-ion battery, electrode compromises continue at the cathode side of the cell. Again, a nice, layered home is provided for lithium ions to nestle into as the battery is discharged, in the form of a nickel cobalt manganese oxide material. But the metal oxide material adds a certain weight, as well as cost.
Alternate cathode chemistries are under investigation, not least sulfur. ‘We are removing the nickel, cobalt and manganese, which have cost, supply and ethical issues associated with them, and replacing that with sulfur which is basically a waste product available all over the world,’ says Hill (for more on global resource issues, see The lithium rush). ‘That gives us lots of sustainability improvements, but also the performance of these batteries is potentially a lot better.’
For a solid, sulfur is a very light anionic element. ‘They are so light you get much more performance per unit mass – around five times higher on paper,’ says Hill. ‘It’s really attractive in situations where weight is important, and also where cost is important – which is everywhere.’
We’ve tried to use MOFs as a model separator material
One downside of sulfur is that its discharge voltage, at around 2V, is lower than today’s lithium ion technology. As a result, it doesn’t pair well with a graphite anode. ‘Because the voltage is low, you need to store more charge, which would mean you need more graphite,’ Liu says. ‘That’s a losing proposition.’ A lithium metal anode, in comparison, has no such compromise. ‘Lithium metal opens the door to sulfur,’ Liu says.
Technical challenges on the sulfur side remain. One issue is side reactions between sulfur and lithium that form polysulfides. ‘If polysulfides find their way over to the anode, they start to form dendrites on the lithium surface,’ says Hill. ‘That’s the path towards a short-circuit, and the end of your battery.’
To supress this issue, Hill has been working on improved battery separators, the nanoporous membranes placed between the anode and cathode to keep conflicting chemical species apart. ‘We want lithium to go across the separator really fast, and we want essentially nothing else to go through,’ Hill says.
Hill’s research background is in metal–organic frameworks (MOFs). ‘We’ve tried to use MOFs as a model separator material, to understand the property and behaviour, and then translate that into cheap, very scalable, readily available materials,’ he says. The team has developed low-cost carbon-based separator materials where, by controlling the pore size and charge, they permit easy transit for little positive lithium ions while blocking the larger, negatively charged polysulfides.
An additional complication with lithium sulfur batteries is that, when the sulfur absorbs lithium during battery discharge, it swells by about 80%. A slab of sulfur would disintegrate after a few cycles; a more elastic material is required. ‘We have developed a sort of spiderweb network where we use flexible binders to hold nanoparticles of sulfur together, and then the cathode could swell up and contract without falling apart,’ Hill says. ‘The latest one we just reported, working really well, is using cellulose.’ The material proved to have the added benefit of absorbing certain polysulfides, helping to keep them sequestered away from the anode. The team is now incorporating these developments into larger batteries, a little bigger than an smartphone battery, Hill adds. ‘We used the battery to power a drone, to show we can do something practical at scale.’
Ten years ago, we got excited when we made a cell we could charge and discharge 10 times
In Liu’s lab, flexible sulfur cathodes are also under development. ‘One approach we have taken is anchoring sulfur to a conjugated polymer backbone,’ Liu says. ‘To make it, we just heat up a mixture of polyacrylonitrile, an industrial textile precursor, with elemental sulfur.’ At around 350°C, the inexpensive starting materials react when the sulfur dehydrogenates the acrylonitrile to form a sulfur-containing, electronically conducting polymer with a polypyridine-type backbone.
‘The material works beautifully, but the structure is extremely complex,’ Liu says. The team is working on characterising it to further optimise its performance. ‘A lot of people in the community are trying to understand this structure because it could be the key to a sustainable cathode material,’ he says.
The 2021 failure of UK lithium–sulfur battery start-up Oxis Energy is a reminder of the challenges that remain, yet progress is being made. ‘Ten years ago, when we first started in this area, we got excited when we made a cell that we could successfully charge and discharge 10 times,’ he says. ‘By changing the structure of the sulfur cathode, and working on the separator, we just published an article where we hit 1000 cycles.’ That same battery is still running in the lab and recently clicked over 10,000 cycles, Hill adds.
As light as air
Theoretically, the ultimate high-energy-density batteries would have a cathode chemistry based on air. Rather than carry around an anionic element such as sulfur, lithium air batteries draw in oxygen from the air as required. ‘The long-term, very high-risk, high-reward battery technologies, are metal–air,’ says Yuzhuang Li, a researcher at University of California, Los Angeles, US.
In terms of energy density by volume, lithium–air is the only energy storage comparable with gasoline
These batteries combine a lithium metal electroplating process at the anode side, with an electrocatalytic oxygen evolution/reduction reaction at the cathode. ‘You have two difficult problems spliced together in one system, and that makes the complexity exponentially more difficult,’ Li says.
Mohammad Asadi at the University of Illinois, US, along with collaborators at Argonne National Laboratory, is among the researchers working on the problem. ‘In terms of energy density by volume, lithium–air is the only energy storage comparable with gasoline,’ Asadi says. ‘But it is not trivial to get there,’ he adds.
Asadi’s recent focus has been a solid-state electrolyte for the lithium–air battery. ‘Once you have a solid-state electrolyte, achieving high energy density is all about the thickness of the electrolyte,’ he says. ‘Low width materials increase cell performance while lowering weight.’
A good solid-state electrolyte must combine mechanical strength, good ionic conductivity for lithium ions, and good connectivity to the anode and cathode. Some researchers have experimented with polymer electrolytes, which have very good electrode connectivity but limited ionic conductivity. Others have tried ceramics, which have very high ionic conductivity but make poor interfaces between the cathode and anode. ‘The challenge is to take the best properties of both types of material, which is how we designed our latest electrolyte,’ Asadi says.
Simply mixing ceramic and polymer components generally leads to the worst of both worlds. ‘So we use a sort of intermediate phase material,’ Asadi says. After a lot of experimentation, the team made a material consisting of a silane polymer, chemically bonded with Li10GeP2S12 nanoparticles. The lithium-rich nanoparticles offer pathways for lithium ions across the cell, improving ionic conductivity, while maintaining good interfaces with the anode and cathode.
‘But what was surprising for us was the chemistry of the cell,’ Asadi says. The typical chemistry in a liquid electrolyte lithium–air battery involves a two-electron transfer reaction which makes lithium peroxide, Li2O2. ‘Our new solid electrolyte interface surprisingly makes reversible four-electron transfer feasible,’ he says. By transferring four electrons rather than two, the battery’s energy density should be significantly enhanced.
The challenge is not necessarily at the lab scale
The key seems to be that the solid–solid interface at the cathode has high enough ionic and electronic conductivity to break O2, forming Li2O, in a four-electron transfer room temperature process. ‘Our battery ended up having an energy density of around 700Wh per kg. We are shooting for 2000Wh per kg – that is the ultimate goal,’ Asadi says.
The team is also exploring whether, in the shorter term, their solid-state electrolyte could boost energy density of more conventional lithium-ion batteries. ‘It is not easy to achieve, but there is promise,’ Asadi says.
Jumping the gap
There’s no shortage of promising avenues for next-generation battery development. ‘In my experience, you see a lot of news media saying “This is the next battery breakthrough”, but I think the challenge is not necessarily at the lab scale,’ Li says. ‘I think a lot more support could be put into bridging that gap between single cell batteries that work really nicely at lab scale, and a real scale battery pack,’ he says.
Furthermore, the establishment of battery ‘Gigafactories’ around the world, based on today’s lithium-ion technology, may make it harder for new technologies to break through, Li adds. ‘You have this huge infrastructure investment, so if your technology is not going to integrate nicely with all these expensive pieces of equipment, it is going to be very challenging to make a dent.’
But for Hill, the sheer accelerating demand for batteries, for diverse applications, is a key consideration for how next-generation battery development will play out. ‘We’ve always had a number of different battery chemistries used at scale – we’ve had lead acid, nickel, lithium-ion, they all coexist,’ he says. ‘There doesn’t have to be only one chemistry, there might be three, there might be five, that each find their niche.’
James Mitchell Crow is a science writer based in Melbourne, Australia












No comments yet