James Mitchell Crow explains how chemists are turning a problematic greenhouse gas into commercially useful molecules, at industrial scale
Life on an island alive with active volcanoes can have its downsides. But it can also have many benefits. Iceland, an island country located where the North Atlantic meets the Arctic Circle, is in the enviable position of getting all its electricity and district heating from renewable geothermal, hydro and wind energy.
Given the complete absence of fossil fuel power stations, Iceland might seem an unlikely spot for the world’s first facility for recycling CO2 emissions into fuel. But that’s exactly what Icelandic company Carbon Recycling International (CRI) has been doing for the past five years.
Thanks to the geochemistry of hot rock, CO2 naturally vents into the atmosphere from geothermally active areas. It’s also dissolved in the local groundwater. When this free source of hot water is channelled through a geothermal power plant, the result is a concentrated stream of CO2 that is ripe for capture.

To the captured CO2, CRI adds hydrogen over a heterogeneous catalyst, making methanol. The company sells this versatile molecule – at a premium over fossil fuel derived methanol – for use as a renewable fuel and chemical feedstock (see Hydrogen economies).
CRI is far from the only company committed to making commercial products by recycling CO2 emissions. Even big industry players such as Covestro– formerly Bayer MaterialScience – are embracing the idea. A rapidly expanding number of academic labs, meanwhile, are exploring new ways to rehabilitate the troublesome greenhouse gas – and showing there is a surprisingly rich chemistry to be had from what was long-dismissed as a dead-end molecule. Far from a compromise, designing chemical production around using CO2 as a feedstock can have advantages over traditional routes.
Catalysing change
University of Oxford, UK, chemist Charlotte Williams had been developing ways to make green polymers from plant-derived materials when the idea of using CO2 as a polymer feedstock came to her in the early 2000s. ‘I became fascinated by the idea of using CO2 as a resource because it overcame some of the problems you see with a typical biorefinery,’ she says. Solid plant material can be quite a challenge to process. ‘But CO2 is a gas, so its properties and physical chemistry are much more similar to monomers that are used today to make polymers. I thought what an interesting idea to take CO2 and try to put that into the polymer backbone.’
The other appeal, she adds, was the sheer challenge of trying to do chemistry on a molecule that is the thermodynamic endpoint of many processes including combustion and metabolic processes. CO2 is what you’re left with when you take a hydrocarbon and wring out all the energy. Turning it back into something useful is an uphill battle. ‘But this is a great opportunity for catalysis, to find pathways to reduce the activation barrier,’ Williams says. ‘Currently there is rather a limited range of catalysts that are effective.’
One CO2 catalysis project William is working on is a long term collaboration with chemists and engineers at Imperial College London, UK, making CO2 into methanol. Like CRI in Iceland, the team aims to use renewably generated electricity to electrolyse water, then combine the resulting hydrogen with CO2 through catalysis to make methanol. Their point of difference is the copper zinc oxide catalyst they have developed.1 ‘What’s rather different about our approach is we’ve come up with well-defined ways to make very small nanoparticles, stabilised as colloids,’ Williams says.
One advantage of using stabilised nanoparticles is that you maximise the surface area of the catalyst available for driving the reaction. The system’s other appeal is that by modifying the nanoparticle’s synthesis, it is possible to alter – and hopefully optimise – the interface between the catalyst’s two components. ‘That’s the hypothesis we’re working on – that finding better ways to control the interface between the copper and zinc oxide will allow you to improve efficiency,’ Williams says.

Christian Doonan at the University of Adelaide in Australia and his colleagues have applied a similar philosophy to improve a closely related reaction, the catalytic conversion of CO2 and hydrogen to make methane gas.
A metal organic framework (MOF) chemist by background, Doonan had been working on ways to use these porous materials to capture CO2 emissions when he was drawn into the CO2 recycling side of the story – and he suspected MOFs could help here, too.
‘The commercial catalysts for CO2 methanation are made of nickel. But nickel has issues with lifetime, stability and poisoning,’ Doonan says. In the lab, ruthenium-based catalysts on a metal oxide support have proven to be very stable and active catalysts for the reaction, he adds.
Despite the name, support materials do more than hold the catalytic metal in place. They’ve been shown to play an active role in the catalytic process. ‘The theory is that there will be site defects on the metal oxide that activate carbon dioxide molecules and facilitate catalysis by the ruthenium nanoparticles,’ Doonan says.
The only current downside to these catalysts is the high cost of ruthenium. ‘We thought, could you develop a porous metal oxide catalyst that uses less of the ruthenium?’
The answer? Yes and no. Although the material the team got by combining ruthenium with a zirconium oxide based MOF wasn’t actually porous, it was a highly effective CO2 methanation catalyst,2 even when 1wt% of ruthenium was used. The MOF’s surface acts as a template for the formation of ruthenium nanoparticles, highly dispersed across the surface. The result is that essentially all the ruthenium is exposed on the surface and available for catalysis. ‘MOF templating gives you a very unique structure you can’t achieve by any other synthetic route,’ says Doonan. A back of the envelope calculation suggests you could make about 168 tonnes of methane a week from a catalyst containing 75g of ruthenium, he adds.
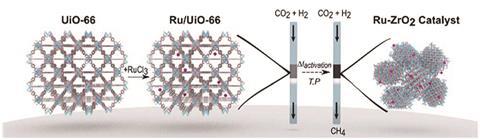
One avenue to explore is the possibility the same templating trick could work for other catalysts. It could slash the cost of the palladium catalysts the petrochemical industry uses for hydrogenation reactions, for example.
And the team is already in talks with prospective industry partners to develop their CO2 methanation process. Although the catalyst works well with pure CO2 and hydrogen, with no dropoff in activity after 200 hours, one of the companies Doonan is in talks with is keen to collaborate on a project to test the catalyst on real world gas streams.
Life imprisonment
Though incorporating CO2 into a molecule that’s burned as a fuel offsets the fossil fuel that would otherwise be burned, the CO2 is soon out into the atmosphere again.
A more permanent solution would be to incorporate the CO2 into a chemical that could be used as a feedstock for making materials. And there’s another reason for taking this approach to CO2 recycling – feedstock chemicals command a much higher price than fuels. ‘My opinion is you have to be able to make chemicals competitively before you can think about making fuels competitively because chemicals are much more valuable than fuels,’ says Matthew Kanan, a synthesis and catalysis chemist at Stanford University, US.
Not that making chemicals from CO2 is easy. ‘I teach organic chemistry, and we teach that you can take a Grignard reagent or an organolithium reagent and combine that with CO2 and make a carboxylate,’ Kanan says. ‘From the synthetic chemistry perspective that’s a fine method for making a carboxylate, but from a CO2 utilisation perspective those methods are totally worthless.’ The energy and emissions involved in generating these reagents would be so high they would completely negate the benefit of using CO2.
So Kanan has tried to come up with the simplest possible way to achieve this carbon–carbon bond forming transformation with CO2.3 ‘What we landed on was, if we could take C–H bonds and deprotonate them with carbonate, a really abundant base with a small footprint, then we could generate a carbanion we could trap with CO2,’ he says.
And the chemistry works. ‘The trick is to get away from solution phase chemistry,’ Kanan says. Rather than using a solvent, the team runs the reaction in a mixture of alkali metal carbonate salts heated to around 200°C. ‘In that medium the acid-base properties of molecules are totally different to what you observe in water or organic solvents,’ he adds. ‘You are able to do a lot of deprotonations you couldn’t do otherwise.’
The application the team has developed the furthest is the synthesis of furan-2,5-dicarboxylic acid (FDCA), a bio-based molecule with many applications including as a feedstock for polyethylene furandicarboxylate (PEF), a green substitute for the very widely used non-renewable polymer polyethylene terephthalate (PET).
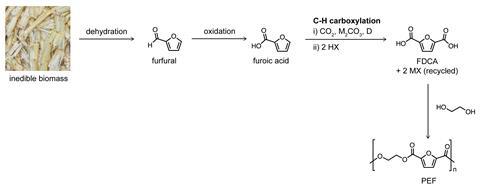
At the moment, FDCA is made from fructose, via a ‘pretty nasty oxidation step’, Kanan says. His alternative route takes furoate, a molecule produced industrially from lignocellulosic plant waste, and adds CO2 to give FDCA.
‘If your target is a commodity scale carboxylic acid, most of the methods for making those involve oxidations – and those oxidations are a liability, they are expensive because they are typically under very corrosive conditions,’ Kanan says. ‘There are many reasons why the chemical industry would like to avoid those oxidations if they had an alternative.
‘That’s where we think there’s the first major opportunity. If you can provide routes to carboxylic acids and dicarboxylic acids where you’re using a C–H bond and CO2 and carbonate, then regardless of whether you care about carbon footprint or not it could be just a really compelling synthesis of those targets compared to what’s done today.’ Kanan’s team is currently in discussions with several potential industrial partners.
When Williams first moved into CO2 reuse research in 2003, incorporating the molecule into the carbon backbone of a large scale commercial polymer was also her initial aim. Rather than PEF, Williams’ target was renewable polycarbonate polyols, valuable polymers used to make polyurethanes.
Traditionally, polyols are made by the ring-opening polymerisation of fossil fuel derived epoxides. Williams and her team came up with a catalyst that could form a polymer in which every second monomer in the polymer chain is a CO2 molecule.
‘One of the things that we’ve found to be a successful strategy has been to develop what we call dinuclear catalysts,’ Williams says. ‘These are homogeneous catalysts that contain two metals in close proximity to one another.’ Inexpensive metals such as zinc and magnesium work well, and the best catalysts are the ones that contain one atom of each metal. ‘We’ve carried out quite a lot of kinetic studies over the years and we believe both metals are integral in the catalytic cycle – the polymer chain shuttles between the two metals.’
Not only is the catalyst efficient. Just as importantly, it works at low pressures of CO2, even as low as 1 bar. That makes it compatible with the existing infrastructure for making polyols, and far simpler to retrofit. In 2011, Williams formed a spin-out company, Econic Technologies, to commercialise her polyol catalyst technology.
‘We’re working with a number of partners in the industry,’ says Rowena Sellens, company chief executive. ‘There are others in the industry area working on CO2 based polyols, but one of the unique features we offer is that ability to retrofit.’
The chemical industry doesn’t really know what to do with CO2, but that’s the job of chemists
Matthew Kanan, Stanford University
The company’s other advantage is the unique ability to tailor the amount of CO2 incorporated into the final polymer, Sellens argues. Williams’ original catalyst system gave maximum CO2 incorporation, with every other monomer unit in the polymer chain replaced by CO2. That soaks up the most CO2, but introduces the biggest change to the original polymer’s properties. ‘We’ve evolved the latest generation of the technology where you can actually tune the amount of CO2 content, so you are able to tailor the polymer properties for the application,’ Sellens says. ‘We believe that will give a much greater reach into the market.’
Econic hopes its industry partners will take their first products to market within the next two to three years, lowering carbon emissions. ‘For every tonne of CO2 you use, because you need to use less propylene oxide you are avoiding 2 tonnes of CO2 emissions,’ says Sellens. ‘I think that’s a really powerful argument for this technology.’
Get just one such CO2 recycling processes up and running in industry, and the floodgates will open, Kanan argues. ‘If anybody can pull that off I think it would really change the mentality in industry,’ he says.
‘The chemical industry don’t really care what their feedstock is,’ Kanan adds. ‘They grew up on hydrocarbons and got really good at processing that. They don’t really know what to do with CO2 but that’s the job of chemists, if we can show them what to do they are brilliant at scaling things and making them hyper-efficient.
‘I’m hopefully someone in the community can demonstrate it is possible to use CO2, and to make a very compelling synthesis, something that’s better than what can be done with traditional methods,’ Kanan says. ‘That would be a real watershed moment.’
James Mitchell Crow is a science writer based in Melbourne, Australia
Hydrogen economies
A growing number of companies around the globe are beginning to recycle CO2 emissions into fuels, typically methane or methanol.
Carbon Recycling International was one of the trailblazers. ‘CRI was founded by four entrepreneurs, two Americans and two Icelanders, in Reykjavik in 2006,’ says chemist Ómar Sigurbjörnsson, who directed the company’s research and development until 2016 before becoming director of sales and marketing. ‘They realised methanol is a very good molecule to make from CO2. We have everything in one place that you need to make this happen.’
The plant uses renewably generated electricity from the Iceland grid to electrolyse water into oxygen and hydrogen. The latter is mixed with CO2 from the geothermal plant next door, directly hydrogenating the greenhouse gas – with the help of a proprietary catalyst – to make methanol. The current plant produces 4000 tonnes of methanol per year .
Although CO2 is essentially free, hydrogen can be expensive, thanks to the energy needed to split water. ‘The main cost driver for our process is the cost of electricity for hydrogen,’ Sigurbjörnsson says.
One option is to try to run the electrolysis plant at times electricity prices are at their lowest. As the world adopts ever-more renewable electricity generation such as wind and solar, with their inherently fluctuating electricity supply, hydrogen production could become an attractive way to put excess power to good use at times when supply exceeds demand (and electricity prices dip).
In Germany, Audi is already doing just that . The company sells cars that run on ‘e-gas’, a renewable methane the company makes in Werlte, north west Germany from CO2 and hydrogen, which is mainly produced when there is an excess of wind energy in the grid . Audi says it makes enough e-gas to fuel 1500 e-gas cars up to 15,000 kilometres per year.
Alternatively, some industrial processes, including steel making and chloralkali plants, can generate hydrogen as a by-product. ‘The hydrogen is difficult to store and transport, so if there is no other use it is often combusted for heating,’ Sigurbjörnsson says. ‘By using it for methanol production you can increase its value.’
That’s exactly what CRI hopes to do at its first large scale commercial plant, a
50,000–100,000 tonne per annum facility probably based at a chloralkali plant in China. ‘We are hoping to close the contract for the first commercial plant later this year, so that would be operational some time in 2019 or so,’ Sigurbjörnsson says.
‘When we started there was very little interest in this area but now quite a number of companies are involved in some form of carbon recycling,’ he adds. ‘This is what we want to get across, that the technology is mature enough that today you can deploy it in large plants.’
References
1 S Pike et al.,Catal. Sci. Technol., 2017, 7, 3842 (DOI: 10.1039/C7CY01191A)
2 R Lippi et al.,J. Mater. Chem. A, 2017,5, 12990, (DOI: 10.1039/c7ta00958e)
3 A Banerjee et al., Nature, 531, 215 (DOI: 10.1038/nature17185)













No comments yet