Can scientists overcome enzymes’ fragility and exploit their speed and specificity? Fiona Case investigates
Enzymes, proteins that catalyse chemical reactions, are extraordinary: not only do they offer a specificity that is rarely achieved by chemical catalysis, they accomplish this at room temperature and in water. They have become ubiquitous in food processing, and occupy significant niches in personal and homecare products, speciality chemicals and energy industries. The industrial enzymes market was valued at $4.2 billion (£3 billion) in 2014 and is projected to grow at a rate of 7% from 2015 to 2020 as biocatalysts are developed for an increasing range of chemical transformations and enable bio-based (rather than fossil-fuel-based) materials and fuel production.
Enzymes for energy
Bioethanol and biodiesel are already making significant contributions to energy security and renewable energy targets worldwide. But their production has caused controversy, accused of increasing costs for food-grade oils and competing with food production for land use. Enzymes are playing a key role in alleviating this tension.
Ethanol is mixed with gasoline (petrol) used in automotive vehicles, or used to generate electricity: if it is from a renewable source it reduces greenhouse emissions. Currently most is obtained by fermenting sugars from corn. Alpha amylase enzymes play a critical role converting the plant starches to dextrose prior to fermentation. Bioethanol production is among the largest commercial application of enzymes.
‘There isn’t a viable chemical route for producing ethanol from corn starch,’ says Frederik Mejlby, the industry unit director for oils and fats at Novozymes in Basel, Switzerland. ‘We already had an enzymatic process for the production of alcoholic beverages, so when the biofuel business ramped up, particularly in North America, it was easy to adapt.’
Researchers are working to commercialise new enzymes to open up new sources for bioethanol. Fungal enzymes that break down lignocellulose increase the percentage of fermentable plant material and have the potential to open up new, non-food sources for bioethanol.
Biodiesel is produced from the breakdown of triglycerides and is a direct replacement for fossil diesel. The most commonly used chemical process is a base-catalysed transesterification with alcohol to produce biodiesel and glycerol, but 12 months ago Novozymes introduced a new product for enzymatic biodiesel production.
‘The focus of our development is not reaction speed, enzyme efficiency, but robustness,’ says Mejlby. ‘Currently 90% of biodiesel is made from edible oils such as soy and rapeseed. Our enzyme-based process can use unconventional non-feed oil, lower grade materials from foundries, tallow from slaughter houses or even oily sludge from the sewer system,’ he explains.
These low quality sources tend to have high fatty acid content. ‘The fatty acids and wide range of contaminants are a serious problem for chemical synthesis routes, but no problem for enzymes,’ says Mejlby. Novozymes is working with partners including Viesel of Florida, US, who are producing biodiesel with the enzymatic process.
Transforming chemical production
The potential for biocatalysts in the chemical industry is particularly exciting. Enzyme catalysis can provide stereospecific centres important for pharmaceutical and agricultural chemical production, with no side products or waste.
‘It would be difficult to achieve these reactions with chemical catalysis – perhaps with a lot of effort, a complex multi-step organic synthesis, you could achieve the same product, but it wouldn’t be commercially or ecologically feasible,’ says Markus Pompejus, BASF’s vice president of ‘white’ (or industrial) biotechnology research for North America.
Biocatalysts are also making an impact in larger scale production. Pompejus points to acrylamide production as a prime example for commodity use. The monomer can be made using a copper-based catalyst or using an enzyme. ‘The enzyme catalysis pathway produces significantly less, and less harmful, waste. It is used for production of a couple of hundred thousand metric tons per year,’ he says.
So how do you decide whether to use chemical production via chemical catalyst or biocatalysis using enzymes? ‘Each has pros and cons,’ says Pompejus. ‘When we look at a chemical transformation we consider all the options. Enzymes typically work in limited temperature windows. If the entire reaction process must run at a higher temperature it may not be practical to include a biocatalyst for one stage.’ But there are advantages. ‘Biocatalysts provide chemical specificity,’ he explains. ‘They often enable more preferable reaction conditions and are environmentally preferable, but they are not always available for the desired reactions and conditions.’
Creating new biocatalysts
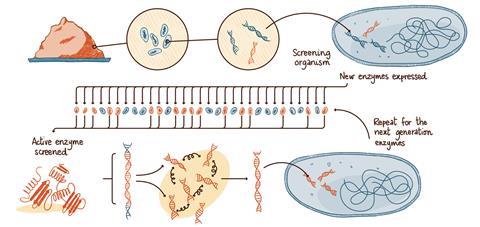
The grand challenge is creating new enzymes for commercially relevant chemistries not found in nature, and for reaction conditions that would be toxic to most cells. The approach introduced by Diversa (now BASF Enzymes) in San Diego, US, in the 1990s, and developed since then, starts with an extensive search for enzymes that have evolved in biodiverse environments.
‘We literally start with a handful of soil,’ says Pompejus. ‘It contains millions of microorganisms. We don’t identify or grow those microorganisms; instead we isolate their DNA, all the DNA, directly from the soil.’
Enzymes in the home
By catalysing the chemical changes involved in cooking and food preparation, enzymes increase the speed, and reduce the cost, of commercial food production. Lipases enhance flavour development and shorten the time for cheese ripening. Asparaginase added to crisps, crackers or biscuits converts asparagine into aspartic acid to prevent acrylamide, a suspected carcinogen, forming during high-temperature baking. Pectinase enzymes used in fruit processing allow manufactures to obtain more and clearer juice, more quickly. Enzymes can also increase shelf life and contribute to sustainability by reducing food waste: if you slice homemade bread it quickly goes stale, but if you buy supermarket bread it stays fresh for several days.
‘Bread goes stale because the starch changes from an amorphous to a crystalline state,’ explains Mejlby. ‘If you toast bread you can reverse some of this phase change, which is why stale bread tastes better if it is toasted, but enzymes added to the dough maintain the water content, prevent the phase change, and keep bread soft and moist.’
Enzymes also provide dough strengthening at lower dosages than traditional ingredients, and improve crustiness. Dutch science company DSM estimates that using enzymes in place of traditional dough emulsifiers such as lecithin can cut CO2 emissions from baking by up to 80%.
Clothing production can be an environmental nightmare with high energy consumption and the creation of toxic waste. Fashionable finishes – such as faded denim and silky-smooth cotton – are particularly egregious. Biocatalysts can help. Cellulase enzymes break down the outer fibrils of cotton, releasing dye molecules resulting in a fashionably faded, soft finish without stone-washing or acid.
When clothes are washed, protease enzymes added to laundry detergent break down biological dirt (such as food and blood), and lipase enzymes break down fats. The fragmented materials can be washed away easily, even with lower temperature washing. The challenge (and motivation for new enzyme design) is getting the enzyme proteins to remain stable in a laundry detergent designed to break down proteins. Many liquid detergents currently use boric acid or borate-based inhibitor systems to shut down proteolysis while the product is in storage, but greater regulatory scrutiny of boric acid and increased consumer focus on sustainable chemistry is pushing detergent makers to seek alternatives.
The DNA is incorporated into screening organisms, typically bacteria that have been engineered to enable the following high-throughput screening procedures. The new DNA instructs the bacteria to create proteins – to build the enzymes originally present in the soil sample. The wealth of DNA from the soil sample creates a library of hundreds of thousands of new microorganisms. Those that create enzymes which catalyse the desired reaction are identified using robotic high-throughput screening systems.
‘We quickly test each clone. We take those that are active and sequence them to find out what the enzyme looks like. Sometimes it is something we have seen before, but quite often is a new enzyme, especially if the soil came from an extreme environment,’ says Pompejus.
For the next step, the genes that created the most successful candidate enzymes can be ‘chopped up’ and allowed to mix. When reassembled and put into bacteria, the next generation can be screened again. Ultimately the enzyme that is produced can include segments of amino acid sequences from radically different environments – there could be a segment coming from deep sea sediments, followed by one from a desert and another one from a jungle soil. The gene-reassembly process can be repeated again and again, pushing towards enzymes that work ever more efficiently under the desired conditions. The process is an accelerated version of natural evolution.
Directing evolution
One of the pioneers of this iterative optimisation is Frances Arnold, a professor of chemical engineering at the California Institute of Technology in the US. She is now using this approach to create enzymes that catalyse reactions not found in the biological world.
‘Natural enzymes didn’t spontaneously appear, they evolved to allow organisms they were part of to take advantage of new conditions or food sources, to occupy new niches,’ says Arnold. ‘Recently, new enzymes have evolved to take advantage of man-made chemicals in the environment, to allow bacteria to eat herbicides or pesticides, for example. In directed evolution we provide a new niche in the laboratory, so to speak, and encourage evolution of enzymes to catalyse commercially useful reactions.’
Arnold starts with enzymes with known activity similar to the reactions she wishes to catalyse. For example, the iron–haem chemistry in cytochrome P450 catalyses a diverse range of oxidisation reactions. The protein structure around the metal centre can be evolved to guide a new reaction down one out of many possible pathways.
‘When we provide carbene and nitrene precursors instead of oxygen we create new pathway possibilities. We direct the evolution using multiple rounds of mutation and screening so that the enzymes catalyse non-biological reactions like cyclopropanation of olefins or direct C–H amination. We have created enzymes for a range of synthetically challenging reactions not known in nature,’ she says.
The rate of natural evolution is determined by the occurrence of random mutations, and this process can be speeded up in the laboratory. Researchers have developed many ways of introducing mutations in the DNA. For example, changes are introduced at specific sites that researchers believe may enhance activity of the enzyme, or error-prone PCR is used to create random mistakes that allow the possibility of improved function.
The new catalysts developed by the Arnold laboratory are being commercialised by a spin-out company, Provivi, to produce pharmaceutical intermediates, agricultural and speciality chemicals. ‘If you find an enzyme that has a little reactivity for a target reaction, you can make it faster using directed evolution. There is plenty of new chemistry which enzymes can do today but no-one ever asked them to do it,’ says Arnold. ‘Now we are asking.’
Designing enzymes
Understanding how an amino acid sequence directs the folded shape of an enzyme, and its function as a biocatalyst, is limited. Directed evolution bypasses this lack of knowledge, reasoning that misfolded proteins will be inactive and thus be selected against, but the proteins that are selected will include a lot of material that is irrelevant for catalytic performance. Leslie Dutton, of the University of Pennsylvania in the US, has been working to reveal the structure–function relationships in enzymes and to design the smallest possible protein structures that are still capable of efficient catalysis.
‘We start with a structurally stable four alpha-helix protein bundle with an interior that can accommodate light- and redox-activated groups such as metal-haems and ?avins,’ says Dutton. ‘Despite its small size, the structure has distinct domains that can accommodate reacting species, and the design allows efficient electron transfer between the co-factors.’
One possible application of the approach is the creation of relatively simple photochemical enzymes, incorporated into the genome of living cells, to provide a self-sustaining way of using sunlight to oxidise water and direct product electrons to reduce carbon dioxide to useful chemicals and fuels.
Designing in silico
David Baker, a biochemist at the University of Washington in the US, approaches the design of biocatalysts from the bottom up. ‘We start with the reaction we wish to catalyse, and use computer modelling to search for and compute the transition state, and to design an ideal active site to stabilise that state,’ he says.
Having designed the reaction site, Baker then sets about designing a protein scaffold to surround it. The first generation of algorithms would search known protein structures to find those that had a pocket that was the right size and shape, with the correct protein alignment.
‘It worked pretty well – but we found that when we embedded the new active site in the protein its structure would change, often in ways that were not anticipated by the design,’ says Baker. ‘We took a step back and decided we needed to build the entire structure from scratch.’
The in silico design of entire enzymes is extremely computer intensive – so Baker is sharing the load with a cohort of enthusiastic volunteers. His original Rosetta at Home program invited people to donate their unused computer time. They had the satisfaction of knowing that their computer was usefully occupied but they didn’t play an active role in the work. Their new Foldit program is more interactive (see Chemistry World, February 2013, p44).
‘It’s a team game,’ explains Baker. ‘Players can direct the course of the calculation; teams compete to design the best protein host for our designed active site. It’s a welcoming community. Teams are always looking for new players and are happy to explain the rules. Players have become very sophisticated protein folders,’ he says.
Baker synthesises the DNA that encodes for the sequences that are predicted to fold correctly. The sequences are cloned into bacteria and the protein is expressed, which is then tested to see if it indeed acts as a catalyst. One application of Baker’s designed enzymes is in the treatment of coeliac disease. Coeliac is a reaction to short peptides (five to 12 amino acids) resulting from the incomplete breakdown of gluten proteins. The designed enzyme has the potential to work in the gut to break down these peptides into smaller, innocuous, pieces.
With applications from simple household products through industrial chemical production to new drugs, the potential for biocatalysis is enormous. ‘We are creating a future where industrial chemistry is carried out by organisms and enzymes,’ explains Arnold. ‘Using sustainable raw materials with benign solvents, and significantly less energy.’
Fiona Case is a science writer based in San Diego, US








No comments yet