'Let's talk after lunch' is the mantra of the microwave chemist, such is the speed at which the technology is uncovering new, cleaner and more efficient reactions. Nicholas Leadbeater reports
’Let’s talk after lunch’ is the mantra of the microwave chemist, such is the speed at which the technology is uncovering new, cleaner and more efficient reactions. Nicholas Leadbeater reports
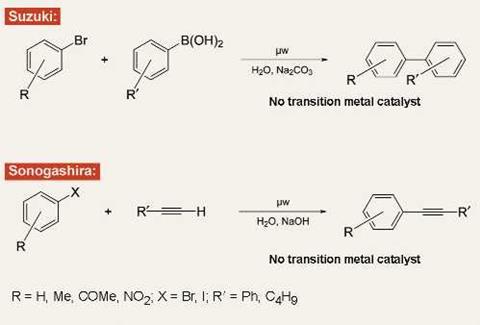
Last March, my group published a paper in Angewandte Chemie reporting the coupling of aryl halides and boronic acids to give biaryls. Nothing too remarkable about that: palladium-catalysed Suzuki coupling reactions are virtually ubiquitous in the modern chemical industry. The difference here was that in our reaction we did not use palladium or indeed any transition metal catalyst at all.
In the past few months, we have again dispensed with transition metals in developing an analogue to the Sonogashira coupling reaction, another key transformation in the organic chemist’s portfolio.
Microwave magic
Our success, and that of an increasing number of academic groups around the world, rests on the technology of microwaves, which are enabling a wide range of reactions to be performed quickly and easily. Among them are allylic alkylations, ring-closing metathesis, cycloadditions, C-H bond activation, numerous rearrangements and reactions directed towards combinatorial chemistry using polymer-supported substrates.
Industry, too, has been quick to spot the potential benefits. Metal-free pathways could slash the huge volumes of toxic waste associated with metal-catalysed reactions. They could also improve the synthesis of fine chemicals, in which heavy metal contamination of sensitive active ingredients can be a setback.
But carrying out microwave reactions in the laboratory is one thing; realising the speed and convenience on a commercial scale is surely something else? No, not at all.
Researchers first reported using microwaves in organic synthesis in the mid-1980s, but the number of applications has burgeoned over the past few years, w ith over 400 publications in 2003 alone. As well as helping us to discover new reactions, microwaves are allowing chemists to perform in minutes reactions that would otherwise take many hours to complete with conventional heating. ’The use of microwaves for our chemistry has led to our mantra, "Let’s talk after lunch"’, says Dean Wilson, medicinal chemistry group leader at Vertex Pharmaceuticals in San Diego. Microwaves quickly allow so many variations of reaction conditions, he notes, that a morning discussion of ’What should we try?’ becomes an after-lunch discussion of ’What were the results?’.
Such rapid feedback allows researchers to develop technologies more easily, in hours rather than days. ’Although some chemists still see microwave heating as the last resort for difficult chemistry, a growing number now believe that it is the first choice for a number of reaction types due to its simplicity and effectiveness’, says Ian Campbell, a medicinal chemist at GlaxoSmithKline (GSK) in Stevenage.
Individual reactions are often more successful and cleaner in the microwave than using conventional heating, report David Benzies, Joe Morrish and Avril Robertson at Tripos Receptor Research in Cornwall. They investigated the alkylation of secondary amines using primary alkyl chlorides and bromides and concluded that microwave heating yields more product for purification.
At GSK, microwaves permit chemistry that would otherwise fail with conventional heating, claims Campbell, citing Buchwald-Hartwig amination reactions. On one occasion, he recalls, a reaction that had been refluxed for 48 hours failed to yield anything until researchers turned to microwaves, which quickly delivered an 85 per cent yield of aminated product. ’Microwave technology is a real chemistry enabler’, agrees Neil Moorcroft, a medicinal chemist at Aventis Pharmaceuticals in Bridgewater, New Jersey, US.
State-of-the-art
What’s behind such successful synthesis? One of the main reasons is undoubtedly new state-of-the-art microwave apparatus. Instead of the equipment designed for heating up coffee or last-night’s leftovers, which makes organic synthesis hazardous and irreproducible, the latest microwave systems allow researchers to control precisely and safely the power going into a sample. ’The technology has proved robust and has been operated on a daily basis with very minimal downtime’, says Campbell. ’The simplicity of the programming and the ease of use of the technique have led to a very wide acceptance of this approach to performing reactions.’
Other benefits include the ability to contain reactions involving ’superheated’ solvents. This allows researchers to choose solvents from which it is easy to isolate products.
’Important instrument innovations now allow for careful control of time, temperature and pressure profiles, paving the way for reproducible protocol development and transfer from lab to lab and scientist to scientist’, notes Dean Wilson of Vertex.
Thinking big
’As microwave chemistry continues to catch on’, notes Moorcroft of Aventis Pharmaceuticals, ’people working in chemical development will start to ask: "can I take this conventional thermal reaction and make it even better, more efficient and cheaper on a tonne scale by using microwaves?"’. At least three manufacturers of microwa ve systems are convinced that they can lift the chemistry from the lab bench into the process plant.
CEM’s ’flow-through’ apparatus pumps the reaction mixture through a U-tube located in the microwave cavity. Using this approach, it is possible to scale chemistry directly without changing or re-optimising reaction conditions, the company claims. Personal Chemistry takes a similar line with its 300 ml ’batch’ vessel, again saying that it is possible to scale up chemistry without re-optimisation. Milestone offers a range of vessels from 20 ml to 1000 ml, all for use in the same microwave unit, for working either under standard open reflux conditions or sealed.
However, neither the flow-through nor the batch approach is without challenges for further development: solid materials or heterogeneous mixtures can cause problems for a flow-through system while, with large reaction vessels of 10s of cm diameter, microwaves might not fully penetrate the bulk material.
Among the hotly debated issues associated with scaling up microwave chemistry is the uncertainty about the existence of ’non-thermal’ microwave effects as opposed to the simple thermal effects or ’specific microwave effects’, which appear to be responsible for accelerating rates of reaction that conven tional heating cannot duplicate.
Special effects
Specific microwave effects do not explain the accelerated rates of reactions in the microwave chemistry of Andr? Loupy, director of the Laboratoire des Reactions S?lectives sur Supports, at Universit? Paris Sud. If the polarity of a system is enhanced from the ground state to the transition state, reports Loupy, it can result in an acceleration of the reaction due to an increase in material-wave interactions during the course of the reaction.
Other groups have proposed that the origin of microwave effects lies in the perturbation of terms in the Arrhenius equation (k = A*exp(- E a/ R* T ), where k is the rate coefficient, A is a constant, E a is the activation energy, R is the universal gas constant, and T is the temperature).
However, from my own experience, I have not seen any effects that cannot be rationalised by efficient heating of the reaction mixture.
Process engineer Mark Lockwood and his team of chemists, physicists and chemical engineers at AstraZeneca are the first to apply thermal testing methods to model the heat profiles of microwave reactions.
In an effort to get to the bottom of the conundrum, the team is evaluating the available software packages that are generally used to predict the behaviour of most conventional reactions ahead of scale up, but whose reliability for microwave chemistry remains unproven.
’In order for the real potential of microwaves to be realised within industry, the hazard management skills of other professions such as chemical engineers need to be utilised’, Lockwood says: ’At a recent course in Austria only one chemical engineer was present in a group of nearly 50 chemists’.
Whether or not non-thermal effects exist, microwave chemistry is a lready making a real impact in the chemical, and particularly the pharmaceutical, industry. ’In a business where time is of the essence, the speed, efficiency, and convenience of microwave chemistry offers undeniable, cost-effective benefits in the race to discover and develop valuable new therapeutic agents’, says Dean Wilson of Vertex.
Converting chemists
The increased availability of scientific microwave apparatus means that more and more chemists are turning to microwaves as the first choice for their reaction.
Watch this space: a few years from now, the microwave oven will have superseded the oil bath as a heating tool. It is safer, more controllable, easier to use and faster, and opens up new and exciting chemistry .
Acknowledgements
Nicholas Leadbeater
Further Reading
- N. Leadbeater et al, Angew. Chem. Int. Ed., 2003, 42 , 1407.
- Microwaves in organic synthesis, A. Loupy (ed), Weinheim: Wiley-VCH, 2002.
- B. Hayes, Microwave synthesis - chemistry
at the speed of light, Matthews, NC: CEM Publishing, 2002. - Microwave-Assisted Organic Synthesis (MAOS) Webpages
- Microwave Chemistry web pages
- Companies: GSK, Stevenage; Tripos Receptor Research, Cornwall; Aventis Pharmaceuticals, Bridgewater, NJ, US; Vertex Pharmaceuticals,
San Diego, US; and AstraZeneca.
Model Systems
The basic microwave ovens offered by CEM (Discover), Personal Chemistry (Creator) and Milestone (MicroSYNTH) are found in a range of academic and industrial laboratories. The CEM and Personal Chemistry instruments are both focused ’monomode’ systems using a 300 W microwave source. The Milestone equipment operates as a multimode system and uses two 800 W microwave sources running at a total of 1000 W. All thre e instruments offer temperature measurement (either by fibre-optic or infrared technology), stirring via a magnetic stirrer plate below the cavity, and pressure measurement.
Discover uses either septum-sealed 10 ml glass tubes or standard glassware (1 to 1 25 ml) at atmospheric conditions; Creator uses septum-sealed tubes of capacity from 0.2-0.5 ml to 10-20 ml; and Ethos uses either standard glassware or sealed glass or polymer reaction vessels that are rotated through the microwave field using a turntable .
More sophisticated add-ons and advanced models are also available. CEM Discover offers a larger capacity (80 ml) vessel and a number of ’bolt-on’ add-on components that allow for automated synthesis and, very recently, peptide synthesis. In addition, CEM is trying to make its Discover unit more compatible with other commercially available liquid handling systems.
Personal Chemistry has developed an automated synthesiser that can run either small or larger volume samples and an automated synthesiser with liquid handling capability.
Milestone has a combinatorial chemistry attachment for its MicroSYNTH apparatus. This consists of a rotor that accepts microtitre plates in 24-, 48- or 96-well configurations. Milestone also has a multiple batch reactor attachme nt for its MicroSYNTH apparatus which can take 6-12 vessels with volumes of 2-270 ml.
Contact and Further Information
Nicholas Leadbeater
Assist ant professor
University of Connecticut, department of chemistry, Unit-3060, 55 North Eagleville Road, Storrs, CT 06269-3060, US







No comments yet