Organic solvents make up a huge part of the waste from the chemical industry. Clare Sansom looks at efforts to reduce the loss or replace them entirely
The pharmaceutical and fine chemicals industries have a problem with waste. Synthesising the complex molecules that these industries produce requires a mind-boggling amount of raw materials, much of which is left behind. And the largest contribution to that waste is the solvent in which organic synthesis is carried out. Solvents account for about 80–90% of the total mass used in any organic reaction, and 80–85% of the waste it produces. The industry depends on solvent-based organic synthesis, but it is difficult and expensive to dispose safely of the ocean of waste solvent left behind.
What can be done? The simplest solution is to re-design syntheses to use less solvent. Pfizer has managed to reduce the amount of organic waste produced in the synthesis of its blockbuster lipid-lowering agent, Lipitor, by 65% by changing the manufacturing process. But this drug is produced in huge quantities, so the remaining 35% – which consists largely of two solvents, methanol and tetrahydrofuran – is not insignificant; and this is just one example among many in the pharmaceutical industry. What about the rest, where the stories have been far less successful? A recent guide to solvents for organic synthesis classed tetrahydrofuran as ‘problematic or hazardous’. It would be better if a completely different approach to synthesis technique – one that can perform reactions such as the crucial cross-couplings, in which carbon–carbon bonds are formed with the aid of a transition metal catalyst such as palladium – without using organic solvents at all.
An organic legacy
The traditional way of doing synthesis has evolved and developed over many decades, but it is not the inevitable one. Bruce Lipshutz, an organic chemist from the University of California, Santa Barbara, in the US, is one of those who has started thinking outside the box. ‘Modern organic chemistry goes back about 150 years, to the mid-19th century,’ he explains. ‘Its principles were established in an era when the petroleum industry was in its infancy and there were no concerns about the availability or toxicity of “exciting” new hydrocarbon-based products such as organic solvents.’ The world is now a very different place, with organic waste a huge problem, and the chemical industry’s part in this is largely caused by its reliance on these same solvents.
Our research group was the largest polluter in the whole of Santa Barbara county – that shamed me
Bruce Lipshutz, UCSB
During the first 25 years of his career as a synthetic organic chemist, Lipshutz used solvents in the way his predecessors had done, without thinking much about its problems or alternatives. He experienced something of a ‘Damascene conversion’ to green chemistry about 10 years ago, triggered when a health and safety officer came to talk to him about solvent disposal. ‘The official told me that our research group was the largest polluter in the whole of Santa Barbara county,’ he says. ‘That shamed me into changing my practices.’ He has spent the last decade doing just that while developing an approach to organic synthesis that could come to be applied widely.
Every organic chemist planning a synthesis needs to make three choices: the solvent in which the reaction is to take, the catalyst and the reaction conditions. Lipshutz’ solution to many of the environmental problems associated with traditional methods came by looking back millions of years, at the way that nature does chemistry. Enzyme catalysis takes place in water, at near ambient temperatures and using only trace quantities of transition metals for catalysis.
Lipshutz and his co-workers have developed a solvent system that allows many common reactions, including cross-couplings, to occur under ‘natural’ conditions: in water, at room temperature, and with only parts per million of transition metal catalysts. This system consists of an amphiphilic surfactant that can self-assemble in water to form micelles in which the catalysis takes place. ‘Self-assembly occurs as long as the concentration of the surfactant is higher than a critical micelle concentration,’ explains Lipshutz. ‘This is very low – typically about 10–4M – and the micelle solution can be stored at room temperature for months, ready for use.’
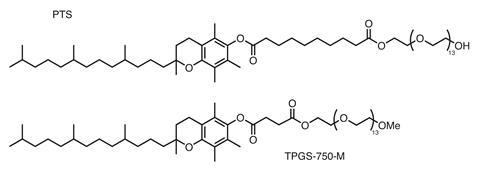
Many organic reactions that need all reagents to be dissolved in a lipophilic solvent can take place in the interior of these micelles, although the surfactant must be chosen carefully as micelle size and shape are critical. If they are too small, most reactions do not go to completion, and if they are too large they form heterogeneous particles in which the chemistry is slowed down. ‘We are looking at a “Goldilocks” phenomenon here, in which the sweet spot of the most effective catalysis occurs with surfactants that form nanomicelles with diameters of about 45–60 nm,’ adds Lipshutz. The surfactants used in his group, most commonly TPGS-750-M and Nok, are environmentally benign – TPGS-750-M is derived from vitamin A – and form micelles with diameters within this range.
Chemists and regulators often measure the sustainability of a reaction using the so-called E-factor, defined as the ratio of the mass of waste produced by a reaction (not including water) to that of the desired product. The typical E-factors of traditional syntheses that are common in the pharmaceutical and fine chemical industries range from as low as five to over 100. By contrast, those of the syntheses published by Lipshutz’ group are almost always in single figures. These reactions also tend to be very clean, given the mild reaction conditions, and thus to produce high yields.
Scaling up
But no synthesis method, however innovative, will have much effect on the overall problem of waste solvent if it remains in the academic labs that generate a tiny fraction of that waste. Water-based synthesis using micelles will only make a significant contribution to sustainability if it can be made efficient and profitable on an industrial scale. While this particular technology is still at an early stage of development, scientists at one multinational pharma company, Abbvie, are exploring its use in synthesising potential drug molecules.
Wilfried Braje, a medicinal chemist in Abbvie’s centre in Ludwigshafen, Germany, explains. ‘Our work started in 2014 when I came across Lipshutz’ research, and we ended up hiring a postdoc to investigate how applicable it would be to our company’s needs.’ The team started by replicating published syntheses and found that the water-based technique produced yields that were at least as high as those using traditional methods. The reactions occurred readily at room temperature and the products could sometimes even be separated by simple re-crystallisation, meaning there was no need for labour-intensive column chromatography.
Braje likes to cite the example of Negishi couplings, which involve coupling an organic halide to an organozinc compound to form carbon–carbon bonds. ‘Normally, this reaction is very sensitive to water: it just stops dead if there is any around,’ says Braje. ‘It seems counter-intuitive to suggest that it might work in a water-based micelle system, but it does – very effectively.’ This and many other syntheses used at Abbvie use a benign, amphiphilic surfactant with the acronym TPGS‐750‐M, originally derived from vitamin E. ‘TPGS‐750‐M has been designed by the Lipshutz group specifically to work in a wide range of reactions,’ adds Braje. ‘Even discounting the reduction in waste, this method gives medicinal chemists an additional “shot on goal” for reactions that failed in organic solvents.’
The next step is to move the technology out of the medicinal chemistry lab and scale it up for process chemistry, from the gram to at least the kilogram scale. Fabrice Gallou and his co-workers at multinational pharma Novartis in Switzerland recently published a paper describing a process-scale amide synthesis in a water-based system using the ‘designer surfactant’ TPGS‐750‐M with a benign co-solvent.1 ‘Gallou suggests that some syntheses that are widely used in pharmaceutical manufacture can take place at a large scale, at room temperature, while shortening the overall reaction time in this way,’ comments Braje.
A precious problem
Solvent waste is not the only aspect of organic synthesis that Lipshutz, Braje and other ‘green chemists’ are concerned about. The catalysts typically used in coupling reactions are second-row transition elements, most often palladium. This element is even named in the citation for the 2010 Nobel prize in chemistry, awarded to Richard Heck, Ei-ichi Negishi and Akira Suzuki for ‘palladium-catalysed cross couplings in organic synthesis’. It has been shown to be more effective as a catalyst than any other transition metal, but there are potential problems with its use: it is rare, and therefore expensive, and it has toxicity issues. Water-based syntheses use less palladium than equivalent ones in organic solvents, but no matter how little is used the metal will still run out one day.

Robin Bedford, a chemist at the University of Bristol in the UK, is investigating alternatives to palladium as a catalyst, with an emphasis on cheaper first-row transition elements and iron in particular. ‘The current spot price of palladium is over $1000 (£730) per troy ounce (31.103g),’ he explains. ‘It is difficult to quote an equivalent price for iron, but it would probably be under a cent per troy ounce; a difference of over five orders of magnitude.’ This must provide an adequate incentive on its own for exploring iron as an alternative, but there is also a toxicity angle. ‘If you synthesise a drug using a palladium catalyst, you have to be absolutely sure that there is no residue left in the active compound,’ adds Bedford. ‘The regulatory requirements for iron removal are far less stringent as it is of course comparatively benign.’
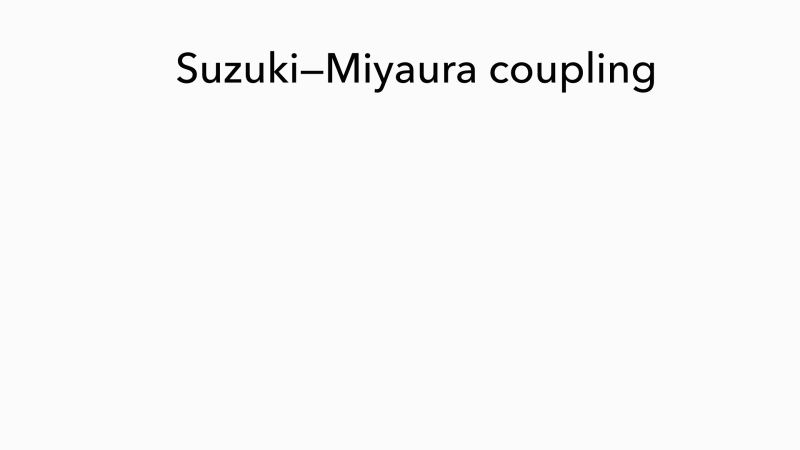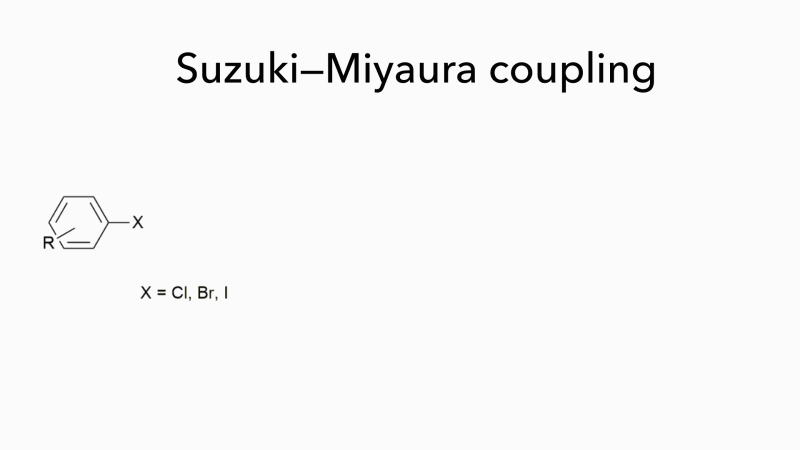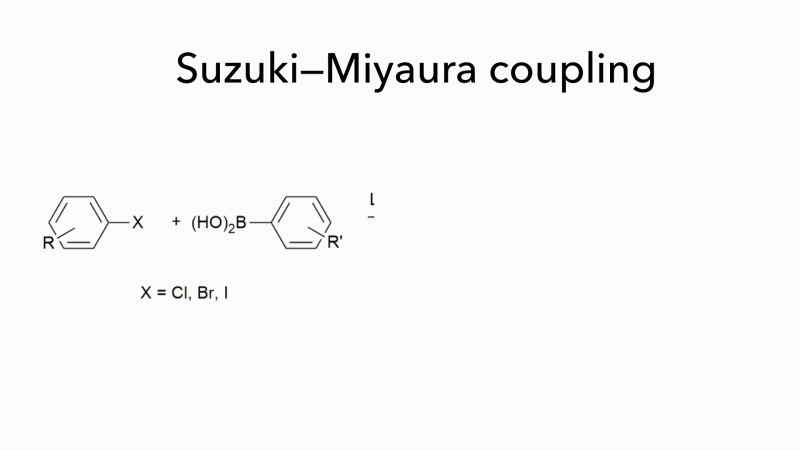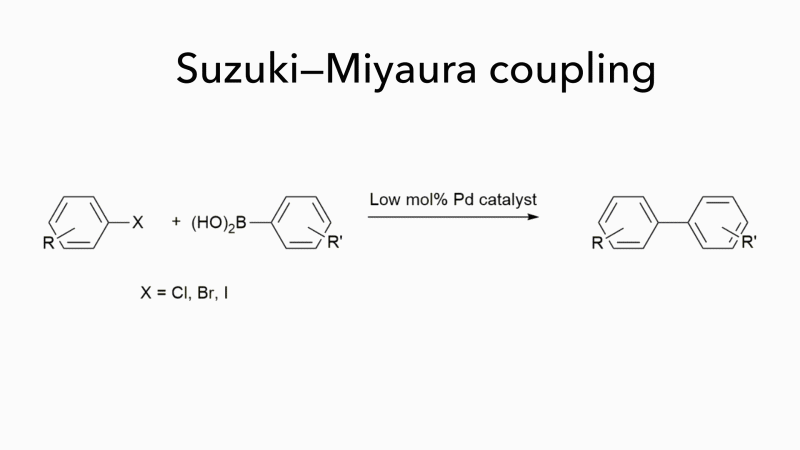
Much of the work in Bedford’s group has concerned Suzuki–Miyaura couplings, which form carbon–carbon bonds by joining a boronic acid to an organohalide and which are often used to form substituted biaryls. These are important molecules, but they fit poorly into the binding sites of most drug targets because they are flat. Bedford is now investigating novel organic syntheses that not only work well with iron-based catalysts but can handle a wider range of reactants, opening up new areas of chemical space and enabling the synthesis of products with more varied pharmaceutical uses. ‘It may seem surprising that a chemistry that uses cheap, easily obtainable catalysts to generate a wider range of molecules with few toxic by-products is not more widely used,’ adds Bedford. ‘It is probably because palladium has been so effective as a catalyst for so long that chemists are reluctant to consider alternatives.’
Going ionic
No solvent, not even water, is appropriate for every application. Many salts that are liquid at or near ambient temperature – so-called ‘ionic liquids’ – can be used as powerful but benign solvents, so much so that they have been tagged ‘green super-solvents’. The study of these materials was pioneered in the 1980s by Ken Seddon, who spent most of his career at Queen’s University Belfast in Northern Ireland, and who died in January 2018 at the relatively early age of 67. Tom Welton, now dean of the faculty of science at Imperial College London in the UK, studied for his PhD with Seddon at the University of Sussex in the UK and became a close collaborator. ‘When I was a student, it was a tiny, obscure field,’ he remembers. ‘In 1985 I attended a Nato summer school with almost everyone in the world who studied ionic liquids [then known as room temperature molten solids] and you could fit them all in a single room.’
The field of ionic liquids is very different today, with thousands of papers published each year and several successful industrial applications. Seddon’s group is best known for developing a process for removing toxic mercury from natural gases by dissolving them in ionic liquids. ‘Mercury is only present in tiny quantities in these gases, but with large volumes of gas you still get a lot of mercury,’ says Welton. Mercury is captured from the gas by dissolving it in an ionic liquid ‘super-solvent’, which cannot, as other powerful solvents might, contaminate the atmosphere through evaporation.
Arguably, however, Seddon’s most important contribution to the field was neither basic nor applied research but the centre he set up at Queen’s a decade or so before these became fashionable. Thanks to his influence, the Queen’s University Ionic Liquid Laboratories quickly became a centre of excellence in both research and education, attracting funding and a wide network of international collaborators and taking in students and postdocs for short-term training.
20 years ago this year, Paul Anastas and John Warner published the ‘12 principles of green chemistry’ that must be considered if chemicals, processes and products are to be developed sustainably.2 The list shows how much impact changing solvents and catalysts can have. Techniques and technologies such as those described here will contribute directly to at least four of these: reducing waste, developing less hazardous syntheses, using safer solvents and reducing the use of derivatives. But ‘ticking boxes’ should not be enough, as Anastas, now director of the Center for Green Chemistry and Green Engineering at Yale University in the US and one of the developers of those principles, explains. Adapting a quote from tech entrepreneur Elon Musk, he says ‘We have no desire to do the best green chemistry. We will do the best chemistry, and it will happen to be green.’ Breaking the habits of a lifetime’s research, changing organic solvents and palladium catalysts for more sustainable ones, and spreading best practices through the sector should help achieve this noble if broad aim.
Clare Sansom is a science writer based in London, UK
References
1 F Gallou et al, Green Chem., 2016, 18, 14 (DOI: 10.1039/c5gc02371h)
2 P T Anastas & J C Warner, Green chemistry: theory and practice, Oxford University Press, 1998











No comments yet