Clever chemistry can turn humble timber into a sustainable material with many uses, Kit Chapman finds
Liangbing Hu can make wood bounce like a ping-pong ball. He can also squish it like a rubber toy, or make it stronger than steel. As he explains from his lab at the University of Maryland, US, it is simple chemistry: using sodium hydroxide and sodium sulfite with a little heat and pressure. ‘You can make all this stuff,’ he says. ‘Wood is a new metal, a new plastic, a new concrete. All you need to know is how to play with it, just by chemical engineering.’
It’s work such as Hu’s that is revolutionising how we look at one of our most abundant and renewable resources – and ushering in a new era of wood technology.
Knotty problems
It has been a long time coming. During the 20th century, wood research was stuck in a rut, says Roger Rowell, a professor of composite agricultural materials at the University of Wisconsin–Madison, US. ‘A lot of wood chemistry has been done before,’ he says. ‘People are going after the lowest hanging fruit, and we’ve had a disappointing lack of innovation.’
Currently, most attempts to innovate with wood has involved transposing it into existing technologies in bulk. In late 2020, Takao Doi, a professor at Kyoto University in Japan – and former astronaut – began to develop wood as an alternative material for building satellites. This, Doi argues, would not only save costs compared with the aluminium and steel structures already in use, but also burn up in orbit once its working life was over without releasing alumina or small pieces of metal as orbiting ‘space junk’ in the upper atmosphere. Doi’s wooden concept satellite, the WISA Woodsat, is due for launch later this year.
Yet this is still thinking too big. Despite an estimated 15 billion trees cut down every year – for lumber, paper or pulp – some researchers argue the lumber industry has largely overlooked the potential of the chemical makeup of its raw material.
The key to understanding wood, Rowell says, is that it’s not a material. ‘If you ask for 404-grade stainless steel, it’s the same the world over. If you ask for wood, every piece is different. It’s a three-dimensional bio-polymer composite. You can’t research wood, you’ve got to look at polymer chemistry, biochemistry, carbohydrate chemistry, lignin chemistry, moisture movement, swelling, shrinking and decay. But to understand wood, to explain its properties, you’ve got to look at it from the inside out.’
We have this technology, but people are still old-fashioned
The chemistry of wood varies depending on the type of tree, but, generally speaking, is relatively straightforward. Wood is largely cellulose and hemicellulose, as well as more lignins, a series of complex organic polymers. These molecular chains weave into elementary fibres, nanofibres, then fibre bundles about 50µm in diameter.
Yet the lack of understanding of what wood is at a molecular level, Rowell argues, means that obvious solutions to major wood problems aren’t being considered. Dampness and rot, for example, is usually caused by fungi. ‘The whole wood preservation industry is based on toxicity, rather than the mechanisms of fungal attack. Nature doesn’t waste energy trying to attack something it can’t – put fungi on glass and it doesn’t do anything.’ Rowell’s research has centred instead on making the wood unappealing. Wood is treated with acetic anhydride, which reacts with plant cell walls to substitute hydroxyl groups for acetyl groups. As a bonus, it’s drier – therefore stronger – and regains wood volume lost through harvesting. ‘Acetylated wood looks like, well, wood!’ he says. ‘It smells like wood. It tastes like wood. But it won’t decay, swell or shrink. We have this technology, but people are still old-fashioned.’
And this is just one option being explored in realising wood’s potential. Other groups are using even simpler chemical reactions and rewriting the rulebook of what wood can do.
Pulp non-fiction
There is nothing old-fashioned about the approaches Hu is taking. Initially, his research focused on carbon nanotubes, before he realised the same principles could be applied to wood. ‘It’s a nanocomposite,’ Hu explains. ‘If you look at wood as a whole, you’d need a PhD in wood science. I’m not interested in those complications. My goal is to amplify the performance of nanofibres inside this big, porous composite.’ Rather than worry about what the whole structure is doing, by focusing on the chemistry Hu has been able to fill his lab with astonishing creations. ‘There are two major categories,’ he says: ‘making wood as dense as possible, which makes it strong … or the other way is to make it as porous as possible, which results in good insulation material and allows you to tune the optical properties … you can pretty much do anything.’
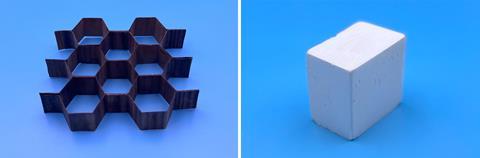
For strengthening the wood, the lignin and hemicellulose in the wood’s structure are partially stripped away using a mixture of 2.5M sodium hydroxide and 0.4M sodium sulfite for seven hours, before being pressed together at 100°C for around a day. This destroys the cell walls of the wood membranes, compressing them and aligning the cellulose nanofibres, reducing the wood’s thickness by 80%. ‘That particular super-wood has strength that’s even better than steel, but it’s six times lighter,’ Hu says. ‘If you tried to break it, you’ll be frustrated!’
If you densify the wood, it’s not only stronger but will take much longer to degrade
The wood isn’t simply load-bearing, either. In 2018, Hu published a paper showing the wood’s possibility as a low-cost, lightweight armour. After shooting a bullet at his densified wood, the team found that it not only resulted in a much smaller projectile hole than you’d seen in natural wood, but it was able to absorb 10 times the amount of kinetic energy. These bulletproof properties increased by adding additional layers with slightly different orientations.
Dense wood has more far-reaching consequences. ‘Think about decarbonisation,’ Hu says. ‘One of the best ways is to just plant more trees. And you want those trees to stay as wood for many, many years – the longer the better – as storage of carbon dioxide. If you densify the wood, it’s not only stronger but more durable against termites and water, and will take much longer to degrade. It can replace steel and concrete. For steel, you get about 1.85kg of carbon dioxide emissions per kilogram. For wood, you remove about 1.8kg.’ Essentially, trees act as giant carbon dioxide stores. Growing wood sustainably to use as a steel substitute isn’t just renewable: it’s better for the environment.
Trunk calls
Conversely, by taking balsa wood, boiling it, then soaking it in the same solution, freezing then freeze-drying it, the lignin and hemicellulose in the cell walls become thinner, with carbonyl and carbon–oxygen bond stretching, but are not destroyed. Instead, they release cellulose fibrils, forming a gel between the wood’s weakened cells that have been transformed into a honeycomb lattice.
The result is squishy wood that bounces and shows no sign of fatigue even after being compressed by 70% more than 10,000 times. In addition, the nanofibrils create channels, allowing ionic conductivity. In 2021, Hu’s team soaked the fibrils in a copper-alkaline solution, then added lithium ions by soaking it in an electrolyte. The result, when made into a battery, performed 10–100 times better than polymer-based electrolytes.
Trees do this kind of stuff every day, right? They pump water
As Hu mentioned, the optical properties of the wood can also be adjusted, simply by altering the cell walls. ‘The wood appears to be white, but on the infra-red, it’s completely black,’ he says. ‘If you put it on a roof, you could cool your house by up to 10°C. It’s a better form of insulation.’ Hu’s work has even developed transparent wood, allowing around 92% of light to pass through.
The Maryland team’s research isn’t just about altering wood to its material extremes, either – it’s also about taking the core properties that nature has already designed, and using them to our advantage. ‘Any type of tree we press becomes the same material. But we have different trees with different microstructures, and some of those have applications for specific combinations.’ Hu is already looking at whether such large-scale structures could be used for desalination. ‘One of the major problems for desalination is that as you collect the steam, you also collect a lot of salt on the surface,’ he says. ‘And that basically kills the machine. But trees do this kind of stuff every day, right? They pump water.’ A hardwood, such as oak, has both large and smaller channels in its trunk. ‘If you cut the wood correctly, you can make sure it’s heated up by solar radiation. The water inside becomes vapour, and you can collect it, and the salt remains inside the wood. Usually, the membrane will start to accumulate salt, but wood, with these big channels, allows the salt to go back into the ocean.’
Despite this promise, the challenge Hu faces is ensuring these marvels can be applied on an industrial scale. ‘We chose these chemicals because they are scalable,’ Hu says. ‘There’s an infrastructure already in place.’ The problem – still to be resolved – is how the chemicals diffuse into the structure. ‘You have a gradient,’ Hu says. ‘If you have enough time to process the wood in the centre, it will damage the fibres on the surface. That’s a major barrier right now.’
Tree dimensional printing
Halfway around the world, Doron Kam at the Hebrew University of Jerusalem in Israel is looking at wood in an entirely different way. ‘It’s a really good question – why look at wood? We’re basically a desert country, we don’t even have woods!’ he jokes. ‘It’s pure science. Wood is an amazing material. It can withstand UV radiation and temperature changes. But if you look around, people are usually taking trees, chopping them down into smaller pieces, and gluing them together. So I began to ask a question: can you build wood from a bottom-up approach, like nature?’

Kam began looking at cellulose nanocrystals. These have already been investigated thanks to their ability to self-assemble into chiral cholesteric liquid crystals, with potential applications such as security paper or mirrorless lasing. They were also easy to make through simple hydrolysis of wood fibres. ‘The end result of the hydrolysis gets these small whiskers about 100–150nm in length and 3nm in diameter, in an aqueous suspension, a colloidal system,’ Kam says. ‘And, as it’s a nanoparticle, we thought it would be a nice resin. The idea is to work with weak binding forces (hydrogen bonding and van der Waal’s) in an aqueous medium without any chemical functionality. Basically, we are gluing wood with wood.’
By using finely pulverised wood-flour, microscopic wood fibres, dispersed in cellulose nanocrystals and xyloglucan (a hemicellulose that exists in cell walls), the team were able to 3D print with wood, with no need for the synthetic polymers typically seen in 3D printing, such as polyvinyl alcohol or acrylonitrile butadiene styrene. ‘We started printing all kinds of objects,’ Kam says. ‘We just started playing around.’
The team began creating simple things: a chess board using two different wood types, a lattice window and a wooden nut and bolt. But this was only the beginning. ‘When you chop down a tree,’ Kam says, ‘the water evaporates out from the log, the different grains have cylindrical asymmetry, and this different orientation gives rise to wood warping. That’s really the main problem for people who sculpt with wood. So we thought “OK, let’s try to mimic this, and then really control it and understand what’s going on”.’ Through this control – and understanding the warping process – the team have been able to better control their 3D prints too, making versatile wood structures of any shape they desire. As wood flour is a waste product from construction, the project is also a prime example of green chemistry.
But there are downsides, as Kam concedes. ‘It’s a really messy system,’ he says. ‘Our system composition is polydispersed, like in nature, as wood flour has a wide range of sizes and shapes, that can vary in tens of microns, and its cellulose nanocrystals differ by tens of nanometres. Right now, we’re just showing that we understand the different phenomena, we can model it, and we can create these objects. We’re focused on fundamental questions, rather than applications.’
It’s difficult to get people whose grandpas worked in sawmills to think differently
There is another issue with taking the technology to market, too. Right now, such printed objects are susceptible to water damage, and likely to lose their strength even after a short amount of exposure. ‘The main challenge, when you play without using any synthetic materials, is water,’ he says. ‘Right now, we don’t know how to make wood-based bio-polymers crosslink nicely without synthetic resins. People are trying to tackle the problem by compressing wood objects, and a lot of groups are looking at different enzymes, but right now it’s a big issue.’
Wood science still has obstacles to clear, then. Once these problems are solved, however, it seems wood – thanks to fairly simple chemistry and basic engineering – is ready for a grand comeback, wowing us in ways that we never considered possible. For Hu, it can’t come soon enough. ‘The world is going to need more materials,’ he says. ‘The good news is that trees, wood, are globally distributed materials – you can use local resources, and process them locally, then we can start addressing global material shortages in our supply chain.’
And yet, Rowell warns, the rate-limiting step to see these applications come to market doesn’t really have anything to do with chemistry. ‘Lumber is an old industry,’ he says. ‘It doesn’t take many chances. It’s all people whose grandpas worked in sawmills, and it’s difficult to get them to think differently. Looking at nanotechnology in wood is 20 years old, and, while we’re starting to do it again, we haven’t really put it into practice.’ And it’s this mindset, Rowell says, that we need to change. ‘We need to start realising that wood is really powerful stuff, that our future materials and chemicals are going to come from it.’
Kit Chapman is a science writer and lecturer based in Falmouth, UK













2 readers' comments