Chemists and materials scientists are adopting a range of three-dimensional imaging techniques to reveal structural secrets. Andy Extance looks inside their work
Chemists and materials scientists are adopting a range of three-dimensional imaging techniques to reveal structural secrets. Andy Extance looks inside their work
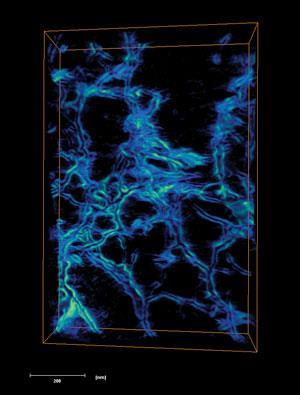
They say a picture is worth a thousand words. But how many molecules and atoms is that? Perhaps a chemist’s most valuable portrait would be of a single molecule, caught at the moment of reaction. Maybe a materials scientist would like to pick up a structure, spin it around and inspect it from all sides. For those lucky enough to work with neatly crystalline materials, x-ray diffraction techniques have been providing detailed three-dimensional images for decades. But as well as excluding less orderly materials – those most often found in the real world – these methods give an average structure that can miss important details.
In such circumstances, biologists have long used three-dimensional imaging techniques, such as electron tomography (ET), x-ray tomography and fluorescence microscopy, to study large biomolecular structures and cells. For over a decade, chemists and materials scientists have been adapting them to yet smaller scales, to provide information on chemical systems like catalysts and biomaterials. And while their efforts have already illuminated previously unseen details, they are still pushing for faster image production, pictures from more ‘real-life’ conditions and higher resolution.
Paul Midgley from the University of Cambridge, UK, and his teammates took some of the first steps towards bringing such techniques into chemistry. ‘We were studying the composition of nanoparticles and nanoclusters in heterogeneous catalysis using electron microscopy, in particular those attached to nanoporous solids,’ Midgley recalls. ‘We felt we were limited by what two-dimensional images could tell us, so my colleague John Meurig Thomas suggested tomography.’
Tomography builds a single three-dimensional representation from two-dimensional scans taken at different angles around an object, an approach well known today in medicine as x-ray computed tomography (CT). CT was commercialised in 1971 by British record label and then computer manufacturer EMI, who (the story goes) used The Beatles’ record sales to fund its development. Meanwhile, researchers were also using electron microscope images for three-dimensional reconstructions, though such electron tomography only became common during the 1990s, primarily in biology.
From the shadows
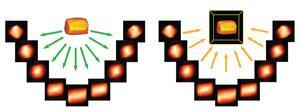
But as the 21st century started, Midgley and Thomas found conventional ET poorly suited to imaging many of their catalysts. Typically it used transmission electron microscopy (TEM) with detectors collecting the sample image placed directly in the continuous parallel beam’s ‘bright field’. But this can complicate the molecular scale picture thanks to diffraction effects in the bright-field image, especially from crystalline structures, leading to blurred three-dimensional tomograms. They also often found TEM quickly damaged their samples.
So Midgley and colleagues switched to scanning TEM, or STEM, where a narrow beam moves over their sample, pixel by pixel, to maintain its integrity. They also used detectors at a more acute angle to the incident electron beam, to record signals in the high-angle annular dark field (HAADF). ‘HAADF gives much stronger contrast and therefore better images than the bright field,’ he says. ‘That took us down to 1nm3 resolution.’
Using electron microscopy, we felt limited by what two-dimensional images tell us
Powered by such annular dark field imaging modes, ET has captured ever more materials over the last decade. Its progress has been driven by automated microscope control, better ‘back-projection’ algorithms used to build the tomograms, and faster computers to run them on. Yet it still faces fundamental limitations, with electron microscopes generally operating under high vacuum conditions that samples would not normally experience. It also destroys samples, slicing them finely to study their composition.
Advances in preparation techniques have reduced the impact such slicing used to have on image quality, according to Lara Estroff of Cornell University in Ithaca, US. ‘The key to electron tomography is a sample thin enough to be electron transparent,’ Estroff says. ‘We use focused ion beam (FIB) milling to prepare them. Though FIB leaves a small layer of surface damage, the bulk of these 200–300nm thick slices haven’t been damaged. You’re capturing largely pristine material at the slab’s centre.’
But such close exposure to beams of high energy ions or electrons can still introduce other artefacts into the images. ‘Irradiating structures can damage them, inducing crystallisation or destroying crystallinity,’ Estroff explains. ‘We’ve seen cases where we initially capture a beautiful diffraction pattern, and on further illuminating the same area under the electron beam, the material becomes amorphous or polycrystalline.’ Knowing the materials being studied is therefore especially important, she adds. ‘It’s always a battle of sample integrity. Part of it is being smart in picking robust materials. One of the best techniques to learn to identify damage is going to a high enough accelerating voltage to see the damage, and then backing off.’
Ultimate sensitivity
Like Midgley, heterogeneous catalysis inspired Maarten Roeffaers from KU Leuven in Belgium to exploit fluorescence microscopy methods that had taken root in biological fields. ‘Most studies treated catalysts as molecular scale factories,’ Roeffaers says. ‘But nobody had a clue what was happening inside the factories, so we wanted to see the chemical processes. With fluorescence microscopy you can see detailed processes going on with single organic molecules, which is the ultimate form of sensitivity.’
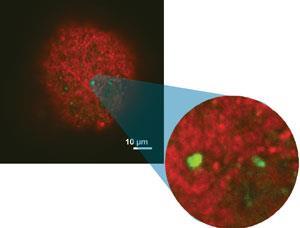
Though fluorescence microscopy can image materials non-destructively, it is usually less sensitive than ET for studying inorganic materials. It detects organic probe molecules that absorb one light colour, produced by a laser, and emit a different colour. ‘This can be very sensitive, because if you illuminate the sample with blue light, and it emits red light, with the right colour filter you can sense only emitted light,’ Roeffaers said. ‘A lens focuses on a certain plane of your sample and only collects information from that plane. Moving focus through different planes gives two-dimensional images from which you can reconstruct the whole three-dimensional structure.’
Bert Weckhuysen from Utrecht University in the Netherlands was drawn into three-dimensional imaging on seeing visualisations from fluorescence microscopy in bioscience applications at a conference. ‘When I saw the very colourful images, it was like opening my eyes on a new world,’ he says. But, like ET, the methods needed adapting to chemistry. Among the tools Weckhuysen needed were probes for staining the catalysts he was studying. ‘Also, we needed better resolution. The protein structures in biology are larger than the features we’re trying to image,’ he says. ‘But if they could do it with cells, why not catalytic reactions?’
Molecule’s-eye view
Thiophene is an important probe molecule for staining zeolite-based fluid cracking catalysts, Weckhuysen’s team found.1 ‘The probe is fluorogenic – it reacts and becomes fluorescent at the active sites,’ says Eelco Vogt, director of R&D at Albemarle Catalysts, based in Amsterdam, the Netherlands, who works with Weckhuysen’s team. ‘That shows how they’re distributed, and if they’re actually reachable.’ Such heterogeneous catalysts are typically made by embedding active complexes in an inert support material so that relatively large oil molecules can reach them. That makes understanding how catalyst particles form, what their pores look like, and how active sites can be reached, highly valuable.
‘Fluorogenic molecules let us see conceptually what a molecule would see going into a catalyst particle,’ Vogt says. ‘We can model the trip that molecule makes, the surroundings it encounters and where it actually reacts. Catalysts are consumed – they deactivate. This can be down to active site or pore blockages, or poisons being deposited. It’s very important for us to understand which it is, and these tools come closer to showing us what’s going on at the atomic level during catalysis. The next step is going to real catalytic conditions, which needs improvements on temperature, pressure and resolution.’
Weckhuysen has worked to achieve realistic conditions with x-ray tomography, which builds up two-dimensional x-ray scans into three-dimensional images.2 In that approach, his team has studied solid catalysts at 10–30bar and up to 600°C in a quartz capillary. But researchers are seeking to make x-ray tomography and other methods, which together Weckhuysen calls ‘five-dimensional microspectroscopy’, even better. Beyond the three spatial dimensions and time, some microscopy techniques can retrieve spectroscopic data, potentially revealing the chemical nature of each pixel recorded.
‘We always want to improve the spatial resolution,’ Weckhuysen says. ‘For example, x-ray tomography is still limited to around 30nm, which is not yet in the range of atoms. We also have to further improve spectroscopic energy resolution to see more chemistry. Data analysis and measurement will also have to speed up to go from frozen pictures to real movies.’
Complementary revelation
Looking at x-ray tomography alongside fluorescence microscopy can build even fuller three-dimensional pictures, Weckhuysen hopes. ‘No one technique will explain everything, but each provides a piece of the puzzle,’ he says. ‘Fluorescence may be less informative, unless you have a very specific staining reaction. But the three-dimensional images that fluorescence microscopy and x-ray tomography produce speak to the imagination.’
Bringing such techniques into new applications always takes extra research, Weckhuysen adds. ‘Every method requires an innovative approach to make it applicable to your own field. Whether it’s a capillary or a staining reaction, it’s not just buying expensive equipment; it’s linking it to your scientific question.’
In fluorescence microscopy, Roeffaers’ team uses furfuryl alcohol as a fluorogenic stain that is converted at acidic sites. In zeolite catalysts, they have revealed 20nm scale features by detecting individual catalytic conversions.3 Such stain molecules help reveal active site accessibility because they must first diffuse towards catalytic sites before becoming visible.

Roeffaers’ team also use reduced fluorescent probes that show active site distribution after being oxidised, with oxidant and probe both diffusing freely inside the catalyst. ‘These complementary techniques allow us to pinpoint catalytic conversions, but also to study sites without needing to consider diffusion,’ he explains. ‘You can find unexpected zones inside a single catalyst particle with different activity, even in seemingly perfect structures.’
As well as catalysts, Roeffaers has used fluorescence microscopy to study metal–organic frameworks (MOFs). He also underlines its usefulness as part of a parallel approach. ‘In catalysis, a lot of expertise in microscopy was focused on the inorganic part, but they didn’t have the techniques to look at the organics,’ he says. ‘Now we are looking for a direct link between organic and inorganic, using electron microscopy and fluorescence on the same crystal.’
Putting power in chemists’ hands

Together with her Cornell colleague David Muller, Estroff has used ET to study the organic–inorganic interface in biominerals, like mollusc shells or sea urchin spines, and their synthetic analogues.4 ‘They diffract x-rays and electrons like single calcite crystals, yet have up to several weight per cent of organic material incorporated into them,’ she explains. ‘The question is: How does the crystal lattice accommodate the flexible polymer? Using ET on synthetic single crystals we were the first to visualise where the organic material was. We then looked at prisms from the calcitic layer of a mollusc using the same technique, and showed that the organics had a preferred orientation. Understanding how the organics are incorporated can help explain both natural and synthetic crystals’ mechanical properties.’
Midgley has applied ET on many different materials, from solar cells to metal nanoparticles that strongly couple to light through surface plasmon quasiparticles. However, he wants to push the techniques further, making them more broadly accessible. ‘People are put off electron tomography because you can need over 100 images, which can take hours to collect,’ Midgley explains. For this reason, his group is developing compressed sensing ET and related methods, using prior knowledge to build three-dimensional representations faster.5 ‘Often you know something about the sample, like gold particles on carbon in vacuum as a simple example. If I know I only have three components, I can use that information to improve the tomogram,’ he says. ‘With these new methods, the number of images and the time needed for experiments can be reduced by an order of magnitude.’
But despite currently being time-consuming, Vogt has already used ET to bring important insights into hydroprocessing catalysts, in collaboration with Krijn de Jong of Utrecht University in the Netherlands.6 ‘We were able to look at individual molybdenum sulfide active particles, 5nm in diameter and 10nm thick, with a resolution of 0.15nm,’ Vogt says. ‘For the first time we had the active particle and could turn it around “in our hands”. That’s important, as chemists are very visually oriented – they always try to make an image of what is happening. That means these techniques will always be of interest.’
Andy Extance is a science writer based in Exeter, UK
References
1 I L C Buurmans et al, Nat. Chem., 2011, 3, 862 (DOI: 10.1038/nchem.1148)
2 I D Gonzalez-Jimenez et al, Angew. Chem. Int. Ed, 2012 , 51, 11986 (DOI: 10.1002/anie.201204930)
3 M B J Roeffaers et al, Angew. Chem. Int. Ed., 2009, 48, 9285 (DOI: 10.1002/anie.200904944)
4 H Y Li et al, Science, 2009, 326, 1244 (DOI: 10.1126/science.1178583)
5 Z Saghi et al, Nano Lett., 2011, 11, 4666 (DOI: 10.1021/nl202253a)
6 K P de Jong et al, J. Phys. Chem. B, 2006, 21, 10209 (DOI: 10.1021/jp061584f)












No comments yet