AstraZeneca strives to foster chemical innovation and evaluate the environmental impact of chemical processes during the manufacture of its medicines. Through purpose-driven academic partnerships, AstraZeneca is developing innovative chemistry to deliver its increasingly complex portfolio while reducing its carbon footprint.
The pharmaceutical industry relies on the supply and delivery of critical raw materials (CRMs) in order to bring vital medicines to patients. These supply chains are affected by global events and while they have almost recovered from the Covid-19 pandemic, the war in Ukraine and geopolitical tensions with China still raise plenty of uncertainty around future global supplies. In addition, the mining of many CRMs has a large environmental cost (from exploration to post-closure) that vastly contributes to the greenhouse gas (GHG) emissions associated with their use.

Among the CRMs on which the pharmaceutical industry is dependent, precious metals tend to be particularly vulnerable to supply chain disruptions, often because their reserves are highly localised. This is problematic as precious metals are among the most utilised CRMs in the discovery and manufacturing of active pharmaceutical ingredients (APIs). Reactions that use abundant metals, particularly first-row transition metals, are less susceptible to supply fluctuations and are generally more environmentally benign. This has spurred academic research towards the development of new methodologies that overcome frequent shortcomings such as low functional group tolerance, poor selectivity or the need for very expensive ligands.
Lowering the quantity of precious metals, or switching to earth-abundant alternatives, can result in significant environmental gains but requires intensive experimentation
James Douglas, AstraZeneca and Univertisty of Manchester
To tackle its environmental footprint, AstraZeneca launched its Ambition Zero Carbon programme in 2019 so far achieving a 59% reduction of Scope 1 and 2 GHG emissions* (equivalent to 380 kilotons CO2 reduction) compared to its 2015 baseline and the value is expected to reach 98% reduction by 2026. The impact will be felt throughout the whole drug manufacturing process, with particular attention placed on the efficient utilisation of (preferably renewable) raw materials and the prevention of toxic or hazardous waste rather than end-of-pipe waste treatment.
To meet these ambitions and inspirational goals requires dramatic change in how we design and develop the manufacturing routes to our current and future medicines, explains James Douglas, a director at AstraZeneca and visiting professor at the University of Manchester. ‘Lowering the quantity of precious metals, or switching to earth-abundant alternatives, can result in significant environmental gains but requires intensive experimentation. To accelerate this, we are investing in high-throughput experimentation (HTE) which can deliver large numbers of high-quality experiments, conducted in parallel, requiring less material, or time per experiment. There has been a highly successful HTE group located in Macclesfield UK for a decade, this recent investment will take that facility to the next level, as well as expanding to our other synthetic chemistry R&D sites in Cambridge, UK; Gothenburg, Sweden; and Boston, USA.’
Recognition of excellence in synthetic chemistry

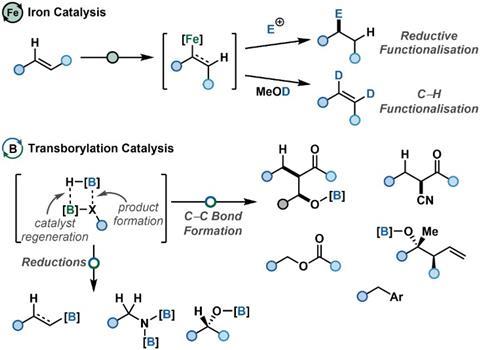
The 2023 AstraZeneca prize in synthetic chemistry has been awarded to Professor Stephen Thomas, University of Edinburgh. Thomas’s prize is in recognition of a very productive early career that has seen the development of new, sustainable methodologies useful to both discovery and development chemists at AstraZeneca. The Thomas group is developing the tools and methodologies needed to enable a sustainable future for chemical synthesis. ‘We have focussed our research in three areas: developing activation strategies to enabling simple access to first-row transition metal catalysis; understanding the reaction mechanisms of iron-catalysed reductive functionalisation reactions; and developing new, sustainable catalytic methodologies using earth-abundant elements’, says Thomas. The sustainability of chemical synthesis relies on catalysis, but this catalysis must now move beyond the platinum group metals. ‘In order to enable the transition to earth-abundant metal catalysis we need easy ways to test and apply these processes and we need to understand their unique reactivity. By studying the reaction mechanisms of low oxidation-state iron-catalysed methods we have introduced a number of activation methods to access these systems without specialist equipment and developed a range of synthetic methods for the formation of C–C, C–N and C–B bonds with direct relevance to pharmaceutical discovery and manufacture.’
Support from companies such as AstraZeneca further highlights the continuing demand for trained synthetic chemists and synthetic chemistry research in the broadest sense
Stephen Thomas, University of Edinburgh
Over the years, AstraZeneca has had a strong commitment to advancing academic research not only through internal schemes but also external collaboration. ‘We are extremely proud of our historical and current support for PhD studentships via industrial CASE awards and centres for doctoral training’ Douglas says. ‘It is vital both industry and academia continue to collaborate, ensuring fundamental advances in chemistry from world-leading UK universities are applied to real world challenges as soon as possible. Furthermore, we believe AstraZeneca has a key role to play in providing the students and researchers of today with the skills and networks required to solve the challenges we will face within the pharmaceutical industry of tomorrow.’
Thomas reflects ‘I have been very fortunate to collaborate with AstraZeneca since I began my independent career at Edinburgh through PhD CASE studentships. This has built an on-going relationship with highly engaged AstraZeneca collaborators, which has underpinned our understanding of what reactions are really needed, the challenges faced during large-scale manufacture and emphasised the role of understanding and controlling reactivity and reaction safety. This type of support from companies such as AstraZeneca further highlights the continuing demand for trained synthetic chemists and synthetic chemistry research in the broadest sense.’ Find out more about career opportunities at AstraZeneca.
Prize-winning lecture
Thomas will be presenting highlights of his group’s work at the annual symposium New frontiers in synthetic chemistry, co-organised by the Royal Society of Chemistry and AstraZeneca, held on 23 November 2023. Both academia and industry thrive on collaboration and the discussion of ideas; alongside the conference lectures there will be a range of engaging break-out sessions including Q&As and panel discussions.
Industrially applied research
For Thomas, part of the excitement of working with AstraZeneca is seeing the research applied to real problems. ‘Ultimately we aim to develop methods that offer new reactivity and sustainable alternatives to current protocols, which enable the exploration of new chemical space.’ he says. The Thomas group has a particular interest in reductive iron catalysis. This requires the use of air and moisture-sensitive catalysts, so an early goal was to find ways to access these catalysts from bench-stable precursors. ‘Our first collaboration with AstraZeneca, a PhD studentship, looked at exactly this and provided the foundations for our use of organometallic and non-organometallic activators. A later PhD studentship with AstraZeneca applied this to manganese catalysis, which started a new area of research we are still exploring’ says Thomas. Working in collaboration with the Neidig group at the University of Rochester, and since 2022 at the University of Oxford, the Thomas Group have built a molecular-level understanding of iron-catalysed alkene hydrofuctionalisation reactions. This understanding was key in developing self-activating iron and cobalt (pre-)catalysts, so greatly simplifying access to low oxidation-state catalysis with these metals.
Our aim was to move beyond the Lewis acid and Brønsted acid modes of catalysis that dominate the p-block and use controlled ligand exchange reactions for catalytic turnover
Stephen Thomas
More recently the Thomas group has expanded out of the d-block and begun a programme of p-block catalysis research. ‘The p-block offers great potential in terms of sustainability. Our aim was to move beyond the Lewis acid and Brønsted acid modes of catalysis that dominate the p-block and use controlled ligand exchange reactions for catalytic turnover.’ Another AstraZeneca PhD collaboration was key to this. The project was focussed on identifying and controlling hidden catalysis, but during the placement the student and their AstraZeneca supervisor realised they had a mechanism to control exchange reactions at boron centres. ‘This has opened a new and very exciting area of research for the group’ says Thomas. ‘We’ve only just started but already we’ve developed a series of catalytic reduction and stereoselective carbon–carbon bond forming reactions, including a novel disconnection for aldol-type reactions.’
Eleonora Fava and James Douglas work in research and development at AstraZeneca and Stephen Thomas is a professor at the University of Edinburgh, UK.
References
P Neateet al, J. Am. Chem. Soc., 2019, DOI: 10.1021/jacs.9b04869
L Britton et al, Chem. Sci., 2022, DOI: 10.1039/D2SC03802A
A Moreno González et al, Angew. Chem. Int. Ed., 2022, DOI: 10.1002/anie.202209584
AD Bage et al, Org. Lett., 2020, DOI: 10.1021/acs.orglett.0c01168
D Willcox and S Thomas, Beilstein J. Org. Chem., 2023, DOI: 10.3762/bjoc.19.28
Additional information
* Scope 1 covers direct emissions from owned or controlled sources. Scope 2 covers indirect emissions from the purchase and use of electricity, steam, heating and cooling.

















No comments yet