Phosphines are well regarded in chemistry as effective ligands within transition metal chemistry due to their ability to tune and enhance catalyst systems. YPhos ligands, invented by the Gessner Research Group, represent the latest step forward in phosphine ligand technology. This novel class of ligands offers users high performance and robust versatility in their catalysis systems.

We have been granted an exclusive interview with Viktoria Gessner, leader of the Gessner Research Group and chair of Inorganic Chemistry at Ruhr-Bochum University, following the acquisition of the YPhos ligand technology by Umicore.
Could you describe your YPhos ligands and what makes them unique, compared to other phosphine ligands available?
YPhos ligands are phosphine ligands that have an ylide substituent rather than an aryl or alkyl substituent as you’d normally expect. This substituent features a carbon atom with a negative charge next to phosphorus, which makes the ligands uniquely electron rich.
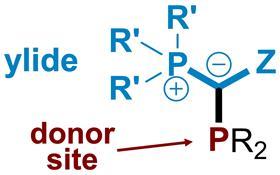
Since these catalysts are more electron rich than the usual alkylphosphines available on the market, there are many catalytic applications that benefit from their use, including both palladium and gold catalysis reactions. Monoligated palladium YPhos complexes, for example, can be used in coupling chemistries, (C-C and C-N bond-forming reactions) and exhibit high activities.1 These reactions are particularly relevant because they’re the kind that are done in the fine chemical industry in the production of pharmaceuticals and agrochemicals.
Additionally, in gold catalysis, YPhos ligands show higher activity in both hydroamination reactions and also in more specialised C-H, C-C bond-forming and cyclisation reactions.2
Frankly, if one is interested in coupling reactions, I would say you should always try the YPhos ligands. Their activity is remarkably high and can facilitate reactions under mild conditions while still giving you the best performance.
What drove you towards this area of chemistry, both in terms of both personal interest and figures that have inspired you?
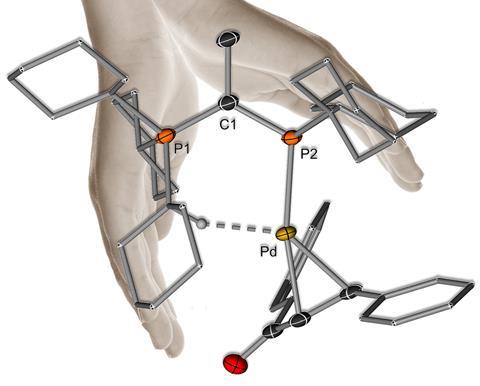
I’m really interested in understanding structures of ligands – and more specifically the structure–activity relationships of them. This is reflected in the YPhos ligands too because, as well as having their special donor properties, the ligands also have a really beautiful architecture. It’s this unique structure that actually gives the catalysts stability by fostering additional interactions between the ligand and the metal. So, it’s not exclusively its donor properties that make them as powerful as they are, but their whole architecture. As we developed YPhos ligands for different purposes it was important that we looked at not only the donor strength but also the steric demands.
In terms of figures who inspire me, Chadwick Tolman really was one of the pioneers who started characterising ligands and really tried to understand structure–activity relationships. He also began giving parameters to ligands and understanding them in terms of coordination chemistry.
More personally, there have been many people I’ve met during my career who have inspired me over the years, including many of my supervisors. My habilitation supervisor in particular, Holger Braunschweig, has done pioneering work in the field of main group chemistry, getting them to act like transition metals, which is something I’ve always really liked the idea of.
It’s my philosophy that happy co-workers are the best co-workers
To develop these ligands, you worked with Umicore. What did you find were some of the benefits that came about by working closely with industry?
The main benefit that I found was getting a different viewpoint into my research. Normally, in academia, you rely mostly on publications from other academics who only sometimes look at the industrial perspective to a piece of research. I’ve found it’s better to have direct contact with someone who really knows about the ins and outs of the current needs of industry and what the environment you’re working to supply is like. This can really help facilitate your development process and help you understand which direction to take your research.
Your team has been responsible for several innovations. How do you facilitate this kind of effective working environment?
It’s my philosophy that happy co-workers are the best co-workers, so I always aim to create a positive atmosphere within the group. In non-pandemic times, we did this by going on regular excursions, for example by doing summer barbecues or going on boat tours. It’s something I really miss during these strange pandemic times.

Separately from this, I also try to create a lot of diversity within the group in terms of research areas. My group is divided into two different types of chemistry: one more focused on reactive compounds and one which investigates catalysis, but I think they can both learn a lot from each other, especially in terms of isolating intermediates and catalysts.
I also ensure that my PhD students have access to all the instruments and good working conditions – dedicating a lot of time to optimising the infrastructure for them as much as I can.
You are well-known for your YPhos ligand technology, but what initially drew you towards chemistry in the first place and how did you end up studying catalysis?
It was clear to me that I wanted to go into chemistry from quite early on. I was always asking ‘why does this work as it does?’ and felt that chemistry was the field that answers this question best. Then during my PhD thesis, I began to notice that research in academia was something that I really enjoyed doing.
I spoke about structure–activity relationships, which I think is something that is really going to change with the advent of machine learning. I am thinking about going into machine learning because that really feels like the future of the field
I obtained my PhD at TU Dortmund after moving with my PhD supervisor from the University of Würzburg. My PhD was initially focused on organolithium chemistry and highly reactive compounds but during my postdoc at the University of California in Berkeley, I began to shift towards supramolecular chemistry. By this point, I was keen to start my habilitation. So, I went back to Würzburg, and after five years I got the call to the Ruhr-University of Bochum where I’m now chair of inorganic chemistry.
My interest in the really academic questions has been a constant throughout my career. Even if these are the things that won’t necessarily go to market or generate profit, it’s always been about solving the really fundamental question ‘why?’ for me and is something I really love to go after.
Where do you see this area of chemistry going in the future?
I spoke about structure–activity relationships, which I think is something that is really going to change with the advent of machine learning. I am thinking about going into machine learning because that really feels like the future of the field. We have this modularity in our ligands and several positions we can change to meet the requirements of the substrates, but it’s not always easy to predict. Machines could change all of this by obtaining all the parameters in terms of both donor strength and sterics. If we can get enough parameters, we will be able to much more quickly develop ligands for specific applications.
This isn’t a hugely new field for chemistry – it started almost 20 years ago in areas like pharmaceuticals, and they have been using it to predict binding properties for many years now. But from the looks of things, some groups in the USA and Europe have begun using it in molecular chemistry, so we’ll probably see more of that in the next few years.
Biography
Additional information
1. See, for example:
- T. Scherpf, H. Steinert, A. Großjohann, K. Dilchert, J. Tappen, R. Rodstein, V. H. Gessner, Efficient Pd-Catalyzed Direct Coupling of Aryl Chlorides with Alkyllithium Reagents, Angew. Chem. Int. Ed. 2020, 59, 20596-20603
- I. Rodstein, J. Tappen, K. McGuire, A. Großjohann, J. Löffler T. Scherpf, V. H. Gessner Palladium Complexes based on Ylide-Functionalized Phosphines (YPhos): Broadly Applicable High-Performance Precatalysts for the Amination of Aryl Halides at Room Temperature, Chem. Eur. J. 2020, 26, 4281
- L. T. Scharf, I. Rodstein, M. Schmidt, T. Scherpf, V. H. Gessner, Unraveling the High Activity of Ylide-Functionalized Phosphines in Palladium-Catalyzed Amination Reactions: A Comparative Study with Cy JohnPhos and Pt Bu3, ACS Catal. 2020, 10, 999-1009
- P. Weber, T. Scherpf, I. Rodstein, D. Lichte, L. T. Scharf, L. J. Gooßen, V. H. Gessner, A Highly Active Ylide-Functionalized Phosphine for Palladium-Catalyzed Aminations of Aryl Chlorides, Angew. Chem. Int. Ed. 2019, 58, 3203-3207.
2. See, for example:
- J. Handelmann, C.N. Babu, H. Steinert, C. Schwarz, T. Scherpf, A. Kroll and V.H. Gessner, Towards the Rational Design of Ylide-Substituted Phosphines for Gold(I)-Catalysis: From Inactive to ppm-level Catalysis, Chem. Sci. 2021, 12, 4329 – 4337
- C. Schwarz, J. Handelmann, D. M. Baier, A. Ouissa,V. H. Gessner, Mono- and diylide-substituted phosphines (YPhos): impact of the ligand properties on the catalytic activity in gold(I)-catalysed hydroaminations, Catal. Sci. Technol.2019, 9, 6808-6815
- Thorsten Scherpf, Christopher Schwarz, Lennart T. Scharf, Jana-Alina Zur, Andreas Helbig and Viktoria H. Gessner, Ylide-functionalized phosphines: Strong Donor Ligands for Homogenous Catalysis, Angew. Chem. Intl. Ed.2018, 57, 12859-12864.


















No comments yet