Inorganic isomerism uncovered in cadmium sulfide clusters
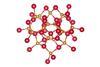
CdS nanocrystals reversibly transition between ‘wurtzite-like’ and ‘zinc blende-like’ forms in a single step
Scientists in the US and Israel have captured a never-before-seen transition in an inorganic cluster – a change akin to isomerisation in organic compounds. The researchers say that the CdS clusters represent a ‘final bridge’ between nanocrystals and molecules.