Natural products – Page 5
-
 Research
ResearchAntimalarial plant's chlorophyll catalyses drug synthesis
New ‘green’ method makes malaria-fighting artemisinin’s synthesis faster and cheaper with industrial production planned for 2021
-
 Research
ResearchEncryption using carbon-13
New technique encrypts materials by altering levels of carbon-13 at regiospecific atoms
-
-
 News
NewsWanted: synthetic chemists (humans need not apply)
Automation could free chemists from tedious lab work – if they’re ready to think differently about research
-
 Research
ResearchOld and new spectroscopic techniques team up to decipher intricate alkaloids
Cutting-edge strategies set to increase our access to chemical space after researchers use them to verify unprecedented structures
-
 Opinion
OpinionNatural selection
Bioactive compounds don’t just inspire new synthetic strategies, they’re also the ultimate test
-
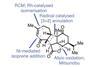 Opinion
Opinion(‒)-Pavidolide B
An innovative approach to making five-membered carbon rings makes for a strikingly short synthesis
-
 Research
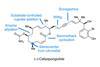
ResearchRoute to fiendishly complex marine molecule cut in half
Shortcut to promising natural product already in Alzheimer’s and HIV trials
-
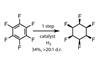 Opinion
OpinionForcing fluorines into shape
Sometimes unnatural molecules can be more challenging to synthesise than natural metabolites
-
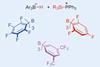 Research
ResearchSuper-electrophilic ions enable selective modification of bioactive molecules
New technique will help medicinal chemists explore chemical space
-
 Opinion
OpinionThat’s stereochemistry
Recognising the tetrahedral nature of carbon changed science as well as explaining it
-
 Research
ResearchStructural sleuthing salvages superbug slayer
Combining ‘assembly line’ synthesis, spectroscopy and computational predictions solves stereochemistry riddle
-
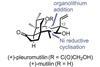 Opinion
Opinion(+)-Pleuromutilin
Total synthesis is sometimes the only way to explore the chemical space around a natural product
-
 Research
ResearchSeductive sulfurs woo wasps
Amorous insect pollinators caught in the act with (methylthio)phenols
-
 News
NewsNews from the American Chemical Society meeting in San Francisco
News stories from the 253rd National Meeting & Exposition of the American Chemical Society
-
 Research
ResearchMaple syrup enhances antibiotics
A phenol-rich mixture extracted from maple syrup allows much smaller doses of antibacterial drugs to achieve the same results
-
 Opinion
Opinion(+)-Zincophorin methyl ester
New and old reactions combine for an elegant and concise synthesis
-
 Research
ResearchTamed radicals expand chemical space
A method to functionalise complex molecules with catalytic radicals could expand chemical libraries of the drug and agrochemical industry
-
 Research
ResearchReactive intermediates tamed in natural product synthesis
Benzynes perform remarkably selective reactions with natural products that lead to uniquely complex compounds
-