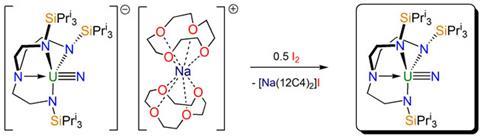
The study, led by Stephen Liddle at the University of Nottingham, builds on the group’s earlier ground-breaking isolation of a uranium(V) terminal nitride. Then, the uranium was enclosed within a bulky ligand, where it was able to capture a single nitrogen from sodium azide. The resulting reactive intermediate was stabilised by the sodium ions, which themselves were subsequently sequestered by crown ethers. The result was a crystalline, separated ion pair, with the anion containing the uranium(V) triply bonded to the nitrogen.
Now, in a surprisingly straightforward process, Liddle and his team has shown that the oxidation of this ion pair with iodine results in a complex containing a uranium(VI). The iodine takes the negative charge from the uranium fragment of the ion pair and pairs up with the positively charged sodium fragment. In one step, therefore, the uranium is oxidised and the sodium counterpart separated. Previously, uranium(VI) nitrides had been seen only fleetingly and at extremely low temperatures.
‘What I have found with uranium is that if something is going to work, it will work really well, or else it will not work at all,’ says Liddle. ‘There seems to be very little middle ground. The key is to have everything just right.’
Other actinide experts are impressed by the work. ‘A 30 year search for a molecular uranium(VI) complex with a terminal U–N triple bond is over and in spectacular fashion,’ says Alfred Sattelberger of the Argonne National Laboratory in the US.
Jacqueline Kiplinger of the Los Alamos National Laboratory, US, also describes the work as ‘spectacular’, and adds: ‘I look forward to seeing how the chemistry of the U–N functional group compares to that observed for transition metal nitrides.’
Liddle’s team will now explore the chemistry of the new compound, comparing, for example, the differences in reactivity between uranium(V) and uranium(VI).
![Structure of [UCl4(HCN)4]](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/5/2/135452_The-structure-of-the--UCl4-HCN-4--molecule-in-its-solid-state-compound.jpg)






No comments yet