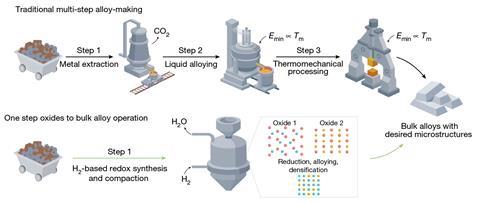
A new way to make metal alloys could drastically reduce the carbon footprint of this important class of materials. As well as eliminating carbon dioxide emissions, the new process offers a direct route to a finished alloy from mixtures of metal oxides, bypassing energy-consuming steps that are associated with traditional means of alloy production.

Alloys are typically made in a multistep process that involves extracting metals separately from their ores before mixing them in the liquid phase at high temperatures. The resulting material usually then requires further heat and mechanical treatment to form a final alloy with the desired microstructure. But this process releases large amounts of carbon dioxide and is highly energy intensive.
Now, a research team led by Dierk Raabe from the Max Planck Institute for Sustainable Materials in Düsseldorf, Germany, has developed a way to convert mixtures of metal oxides into metal alloys in a single step. The oxide species are reduced together by hydrogen while in the solid state.
Traditionally, metal ores are reduced by heating them in the presence of coal. Partial combustion of the coal produces carbon monoxide that acts as a reducing agent to extract the metals from the metal oxides, releasing large quantities of carbon dioxide in the process. To avoid this, Raabe explains that many metallurgical projects have started using hydrogen to reduce metal oxides.
‘But then we realised: what stops us from doing it in that same oven at the same time – the mixing and blending out?’ he says. Raabe notes that usually after the metals required for an alloy have been extracted, they are cooled down, transported to the next facility where they will be mixed together, and heated up again. ‘When you cool it down, you lose all that energy. Then you bring it to the next factory and you have to make it hot – and these metals have melting points of 1400, 1500, 1600°C. We’re talking about a lot of energy that is lost.’
Prized invar alloys within reach
Raabe’s team first performed a theoretical analysis to determine the physical conditions that could enable a one-step oxides-to-alloy conversion. They then demonstrated the process by producing an iron–nickel invar alloy that the researchers describe as ‘one of the most appealing ferrous materials but the dirtiest to produce’. Prized for its high stability, invar is used in many precision instruments. While invar production would usually release significant amounts of carbon dioxide, Raabe’s team claim that their approach ‘maintains a zero CO2 footprint’ – which Raabe says is due to the use of hydrogen as a carbon-free reductant and the elimination of additional processing steps that require further heating.
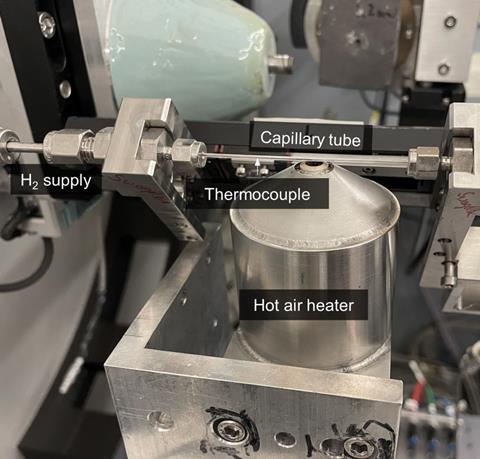
By operating in the solid state, the team’s approach also avoids the very high temperatures that are usually associated with alloy production. ‘In all the traditional ovens, you have to bring [the materials] to the liquid state, to mix it and do all these things – which we do all by solid-state diffusion,’ says Raabe. ‘That means you don’t need these very high temperatures, like 1500, 1600°C to make steel and alloys – we can operate it at 600, 700, 800°C.’
‘The idea of direct alloy production from ore resources … had been proposed by many for quite some time,’ explains Akbar Rhamdhani, an expert on sustainable metallurgy from Swinburne University of Technology, Australia, who was not involved in the project. ‘The approach clearly has the potential to reduce the carbon emissions and energy requirement, especially when hydrogen is used as a reductant.’
However, Rhamdhani points out that Raabe’s team has so far focused on pure metal oxides, which are rare in nature. He also notes that the use of hydrogen as a reductant limits the number of oxides that can be processed. ‘But still there are a number of alloys that can be targeted, eg iron–nickel-based high entropy alloys,’ he adds. ‘The scope of alloys can be expanded, say by using monoatomic or ionic hydrogen as reductants for the oxide.’
Looking to the future, Raabe says he hopes to expand the range of alloys that can be made with the new approach. ‘[We have] started to look at different alloys to see how far can we push that into making very different types of materials that maybe also require different types of microstructures,’ he notes. He also says that the team are keen to adapt the process so that it can work with contaminated feedstocks. ‘Maybe dirty oxides that nobody wants where we can extract valuable metal out of material that you would otherwise throw away from the industry.’
In the short term, Raabe notes that the approach is more likely to be viable for producing high-value alloys that are used in small amounts, rather than for large-scale applications. ‘I already now have several requests where companies want greener metals or metal alloys where the total quantity is not very big,’ he says. ‘I would really target low volume, low quantity things, maybe in the electronic sector, in the electrical sector, in the battery sector, biomedical sector, things like that – and there we often can deal with relatively small chunks of material, where the main incentive would be to make it much, much more sustainable.’
References
S Wei, Y Ma and D Raabe, Nature, 2024, DOI: 10.1038/s41586-024-07932-w
















No comments yet