Amine behind mask hides its reactivity
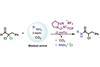
Chemists mask amines’ unwanted reactivity with carbon dioxide and overcome limitations of amide formation

Chemists mask amines’ unwanted reactivity with carbon dioxide and overcome limitations of amide formation