Anionic aluminium turns textbook knowledge on its head
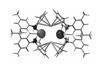
First stable nucleophilic aluminium(I) compound offers new way to make aluminium–carbon bonds
The first stable anionic aluminium compound demonstrates that received wisdom isn’t always right: unlike all other electrophilic aluminium compounds, this unusual molecule is a nucleophile.
Researchers from the UK and Finland made the nucleophile by reducing an aluminium(iii) complex with potassium graphite. Although aluminium vastly prefers to be in a +3 oxidation state – like in aluminium chloride or the polymerisation co-catalyst triethyl aluminium – this reaction creates a bright yellow, dimeric aluminium(i) molecule.
