Assessing covalency in the hydrogen bond zoo
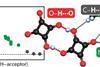
Orbital-resolved contributions provide a fresh perspective on hydrogen bonds with covalent characteristics

Orbital-resolved contributions provide a fresh perspective on hydrogen bonds with covalent characteristics