Atom-swapping reaction turns furans into pyrroles
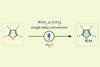
Skeletal-editing technique offers easy way to modify heterocyclic structures
A new photocatalytic strategy allows researchers to swap oxygen atoms for nitrogens, converting furans into pyrroles in a single step. The approach could enable drug discovery chemists to adjust heterocyclic chemical structures with ease, allowing them to study how subtle changes affect the pharmacological activity of target compounds.
‘Existing methods for this type of heterocycle conversion often require harsh conditions, such as extreme heat or ultraviolet irradiation, which limit their applicability in complex organic synthesis due to low product yields and limited substrate scope’, says Mark Levin, an organic chemist at the University of Chicago, US, who was not involved in the study.
