Proposals for the new definition of the tetrel bond will be available for community review in 2025, according to the International Union of Pure and Applied Chemistry’s (Iupac) committee chair Giuseppe Resnati. The announcement follows the release of the pnictogen bond recommendations earlier this year as part of a 20-year mission to formally clarify the terminology around secondary bonding interactions after decades of confusion and misuse.
As a fundamentally non-visual discipline, chemistry needs clear and well-defined nomenclature to provide a reliable and meaningful way for researchers to convey their findings to others. While the hydrogen bond is universally recognised by the chemistry community, its more obscure relatives – the halogen, pnictogen, chalcogen and tetrel bonds – are often overlooked, either misnamed or misclassified as other types of interaction. In recent decades, this widespread misuse of terminology created a disjointed and inconsistent base of knowledge within the literature which, in 2004, Iupac decided to tackle head-on.
As the professional organisation responsible for standardisation in chemistry, Iupac brings together researchers from across the chemical sciences to create a universal scientific language. All recommendations hold a legal status, with feedback and consensus from the wider community playing a vital role in every new project. But as chemistry evolves, so must its terminology, meaning a huge part of the organisation’s role is to challenge existing definitions and conventions which no longer reflect a modern understanding of chemistry.
Naming problems
Secondary bonding interactions are a prime example of this very issue. These weak, non-covalent interactions underpin research areas as diverse as catalysis, supramolecular chemistry and biological chemistry, with hydrogen bonds the most widely recognised example.
For over 60 years, it was believed that hydrogen was the only element capable of forming these bonds and the terms ‘secondary bonding interaction’, ‘non-covalent bond’ and ‘hydrogen bond’ were often used relatively interchangeably. Research in the 70s later identified halogen atoms participating in similar interactions and subsequent studies demonstrated that other p-block elements could also form analogous bonds.
This greater nuance, coupled with the lack of specific names and definitions for these newly discovered interactions created a degree of confusion in the field. ‘People were using this terminology wrongly in the literature or proposing new names and definitions. Different communities were using different terms to say exactly the same thing,’ says Pierangelo Metrangolo, a supramolecular chemist and Iupac officer. ‘We realised there was a need to distinguish each of these interactions clearly.’
Metrangolo, as part of an Iupac committee, has spent the last 20 years working on projects to develop a series of definitions and supporting documentation to help researchers more effectively identify and classify these secondary bonding interactions. Each recommendation includes an explanation of the key features of the bond, followed by a list of characteristic experimental and theoretical evidence for the interaction.
‘People need to have authoritative documents they can refer to where there’s a clear definition, but also a long list of notes where you can get the parameters. If many of the conditions are met, you know you’ve encountered that kind of interaction,’ says Metrangolo.
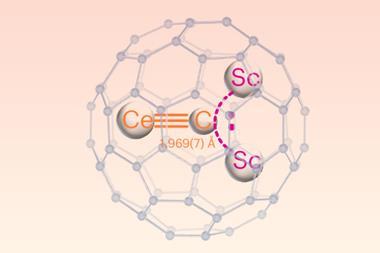
Earlier this year, the team released a definition for the pnictogen bond – the group 15 analogue of the hydrogen bond – with a final recommendation for group 14’s tetrel bond expected in several years’ time. Crucially, this definition, as with others in the series, emphasises the fundamental nature of the interaction. The named donor atom (the pnictogen) acts as the electrophile, creating a predominantly electrostatic attraction towards a nucleophilic region of the acceptor. ‘The aim was to stress that, due to an anisotropic distribution of the electron density resulting from the covalent bonds formed by the pnictogen atom to other atoms in the molecule, some of the outer surface can be electrophilic, even for elements commonly perceived as Lewis bases,’ explains Resnati.
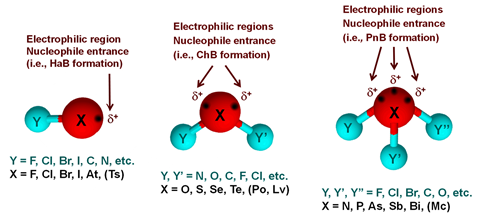
By explicitly naming interactions according to the electrophilic contributor, the new terms enable a clear distinction between similar bonds, which in turn gives researchers a vital tool to influence those interactions. ‘If we can distinguish between a hydrogen bond and a pnictogen bond, we can think about the factors contributing to the force there,’ explains Steve Scheiner, another committee member, specialising in computational chemistry. ‘But many people don’t easily accept the idea that two electronegative atoms can actually form an attractive interaction.’
This lack of awareness is an ongoing issue, with many researchers misclassifying their intermolecular interactions as more familiar hydrogen bonds. However, it was the team’s original work on redefining the hydrogen bond back in 2011 that first revealed the extent of this problem and began to take the first steps to address this systemic miscommunication.
Evolved understanding
The hydrogen bond was first identified over 100 years ago and was originally defined as ‘a weak electrostatic chemical bond which forms between covalently-bonded hydrogen atoms and a strongly electronegative atom with a lone pair of electrons’ – which in practice meant nitrogen, oxygen or fluorine.
However, over the second half of the 20th century, a growing body of evidence suggested that perhaps this narrow definition didn’t tell the whole story. ‘It was considered early on that a CH group was incapable of forming a hydrogen bond. But in the 50s, some crystal structures were published which looked like they had CH–O hydrogen bonds,’ says Scheiner. ‘This was quickly poo-pooed by other crystallographers who argued no matter what it looked like, it couldn’t be a hydrogen bond because it was a CH.’
The spectroscopic criteria for identifying a hydrogen bond were also called into question. An early characteristic sign was red shifting in the IR spectrum – the covalent bond involving the shared hydrogen produced a broader peak shifted towards a lower frequency. However, by 1998, conclusive cases began to emerge where this bond’s frequency was actually shifted in the other direction (known as a blue shift), triggering further disagreement as to whether these interactions could be called a hydrogen bond.
In 2004, Iupac formed a committee to evaluate and refine the existing definition of the hydrogen bond to reflect this contemporary understanding and the team were careful to avoid the limitations of the former definition. ‘When you have to describe a phenomenon you can focus on many key features – the nature of the interaction, the geometric features etc – but what characterises all these approaches is that you make an emphasis of one feature over the others,’ says Resnati who joined the team in 2010. ‘The decision made when we started this was not to focus on the understanding of the interaction which may evolve with time, not on any one feature of the interaction, but on the very essence.’
After seven years, having evaluated an immense body of literature documenting this evolving understanding of the hydrogen bond, the team proposed a new definition alongside a list of criteria and characteristics to support researchers in confidently identifying this type of bond.
‘The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation.’
But, despite the extensive supporting explanation, the new definition initially proved controversial. Ingrained opinions about the nature of hydrogen bonding from across the community made reaching a consensus particularly challenging and ultimately the paper went to over 20 referees before receiving final approval in 2011.
Expanding across the periodic table
It was during the course of this collaborative and cross-disciplinary discussion that the team identified the other key problem with Iupac’s existing definition framework – missing terminology. The lack of clarity around these more recently discovered interactions left researchers to propose their own definitions for these intermolecular bonds, causing confusion and inconsistency throughout the literature.
The term ‘halogen bond’, in particular, was widely misused and in 2010 the halogen bond definition project was proposed, with the analogous group 14–16 project initiated shortly after. At a fundamental level, each of these interactions is part of the same wider phenomenon: the uneven distribution of electron density can create a localised electropositive region, even on an electronegative atom. The resulting electrostatic attraction to a nearby nucleophilic region then creates a (typically) weak bond whose identity is determined by the electrophilic atom.
While the changing valency across the periodic table leads to some differences, the underlying principle behind hydrogen, halogen, chalcogen and pnictogen bonds is the same. Resnati’s team were keen to ensure consistency across this series of definitions, taking the semantic structure of the new hydrogen bond definition as a model for the rest. ‘The extension of the mindset adopted in the new definition of the hydrogen bond to other elements was quite straightforward and reaching a consensus for the halogen and the chalcogen bonds was indeed much easier,’ he says.
The recent pnictogen bond definition proved a little more challenging in this regard as the greater valency and more varied nature of the group’s elements complicated the definition process. ‘In group 15 you move from elements which are typically giving covalent bonds – nitrogen – to elements which are metals – bismuth – so to arrive at a consensus with people with non-minor differences in the concept of their use, bonding and interactions was really tough,’ Resnati explains. The project addressed these issues with the final definition:
‘[A] weak attractive interaction between an electrophilic region on a pnictogen atom in a molecular entity (wherein the pnictogen is involved in other stronger bonds) and a nucleophilic region in another, or the same, molecular entity.’
This additional complexity meant the team spent a total of six years working on the pnictogen bond definition, almost double the time spent on the halogen and chalcogen bonds. However, this effort has not gone unappreciated by the research community.
Clarity at last
Statistics compiled by American Chemical Society (ACS) publications demonstrate the extent to which the chemical community has embraced this new terminology. Following the release of the definition in 2013, halogen bonds were mentioned an average of 73 times a year across all ACS papers, compared with an average of just nine times a year in the 20 years preceding the project’s announcement. A similar trend is also emerging for 2019’s chalcogen bond, with an average of nine times more annual mentions since the recommendation was published.
Clear and specific terminology also streamlines the process of research itself, providing citable terms for researchers to use in publications. ‘It’s simplified my life,’ says Anthony Cozzolino, a supramolecular chemist at Texas Tech University. ‘The definitions help unify the field and give searchable names which, in turn, helps me identify the research that I want to be looking through and target my efforts.’ At the same time, the prevalence of secondary bonding interactions across so many different parts of chemistry brings researchers into contact with other research areas which can then guide new strategies drawn from both fields together.
But more widely, the attention surrounding these definitions has shone a light on these interactions as an important and growing area of research. ‘Having the Iupac definitions lends credence to the field that makes it easier to push in new directions,’ says Cozzolino. ‘In terms of funding, you can show there’s precedent for this chemistry which gives weight to grant applications.’
A huge part of the success of these definitions has been the emphasis placed on consensus – a requirement for all Iupac definitions – and the ACS’s Committee on Nomenclature, Terminology, and Symbols (NTS) is just one of the professional bodies invited to offer feedback on the early proposals. ‘Definitions help us all speak the same language and align around consistent meanings,’ says Clay Harris, strategic initiatives leader for the ACS’s NTS committee. ‘We value opportunities like this to help ensure that proposals work for chemistry practitioners and our global community of members. These positive interactions support the evolution of common terminology used in the global practice of chemistry.’
Resnati’s team are now working on the final definition in the series but the committee expect arriving at a consensus for group 14’s tetrel bond will prove the most challenging yet. The central importance of carbon in so many areas of chemistry, and the contrast with the wildly different properties and behaviours of heavier group members such as lead will likely make the details of the final definition difficult to establish and it could be years before the final definition is published. ‘To arrive at a common opinion shared by chemists with such different backgrounds will be truly challenging but I think that the previous definitions – hydrogen, halogen, chalcogen, pnictogen – are proving that the approach is meaningful,’ says Resnati.
Thanks also to Gautam Desiraju, Anthony Legon and Antonio Frontera for their contributions.
References
Hydrogen bond – E Arunan et al, Pure Appl. Chem., 2011, 83, 1637
Halogen bond – GR Desiraju et al, Pure Appl. Chem., 2013, 85, 1711
Chalcogen bond – CB Aakeroy et al, Pure Appl. Chem., 2019, 91, 1889
Pnictogen bond – G Resnati et al, Pure Appl. Chem., 2024, 96, 135













3 readers' comments