Bubbly protocells bob up and down
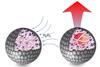
Enzymes inflate or consume oxygen bubbles making clay capsules float and sink reversibly
UK researchers have developed an enzyme-driven protocell that reversibly changes its buoyancy according to its chemical environment, allowing it to move in a controlled way between two points. The capsules could potentially be used to shuttle cargoes from one place to another.