Building block approach creates new metalloproteins
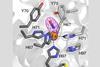
Protein modules can be connected by disulfide linkages while varying the metal ions
A new strategy for engineering metalloproteins – which can be used as a starting point for making new biologically-inspired catalysts – has been developed by chemists in the US. The approach works by joining smaller protein units together to make a large three-dimensional structure with a metal coordination site, which Akif Tezcan at the University of California, San Diego and colleagues show can be done by making simple modifications to some of the amino acids.
