Carbon–boron triple bond formed for the first time in a neutral novel molecule
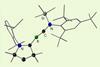
Synthesis could help chemists better understand bonding
Researchers have synthesised a triple bond between carbon and boron for the first time. This discovery could help chemists better understand chemical bonds and inspire other researchers to synthesise compounds that might seem improbable.
