Carbon dioxide recycling on the ball
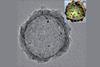
Cocatalysts on the outside and inside of hollow spheres trigger photocatalytic carbon dioxide reduction to syngas
Developing new ways to recycle waste gases into useful resources is becoming increasingly important, but until now, scientists have struggled to turn carbon dioxide into useful products with a good yield. Now, researchers in China have created a new photocatalyst with a hollow spherical structure that converts waste carbon dioxide into syngas, a highly valuable chemical feedstock – and by adjusting the metal composition on the outside of the spheres, the reaction can be fine-tuned to give the most optimal yield of gases.
