Scientists in France have synthesised a thulium complex (Tm(Cpttt)2) that can polymerise carbon monoxide to form multi-carbon oxygenated products.
Whilst its bulky Cpttt ligands stabilise the compound against decomposition, the bent structure of the complex, coupled with the oxophilic nature of the thulium (ii) metal centre, encourages the coordination of small oxygen-containing molecules such as carbon monoxide. Carbon monoxide’s strong C≡O triple bond has historically made this a challenging feedstock to work with, requiring high temperatures and pressures to overcome the activation barrier.
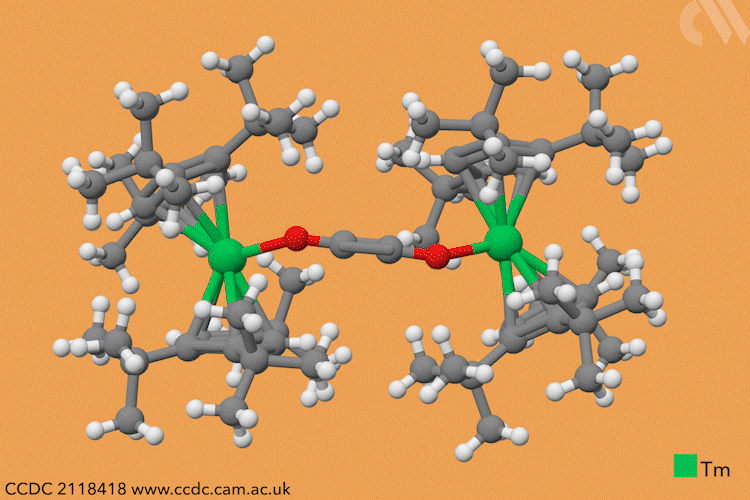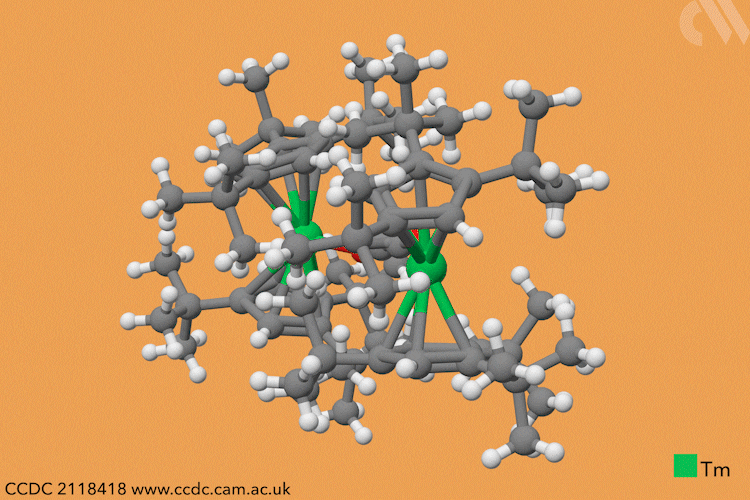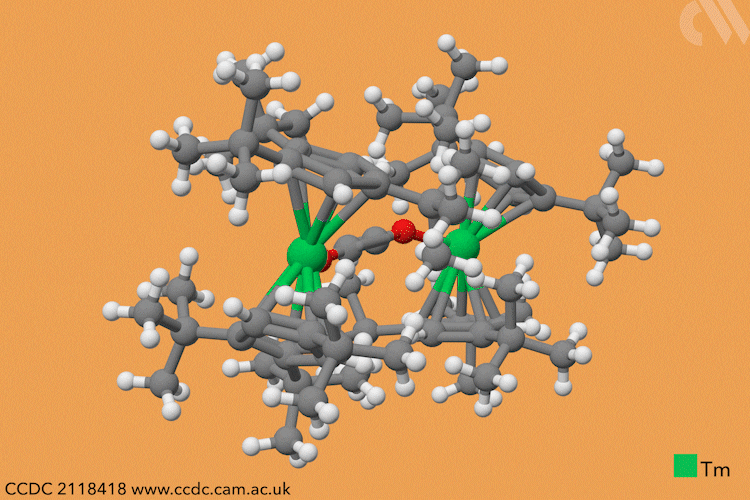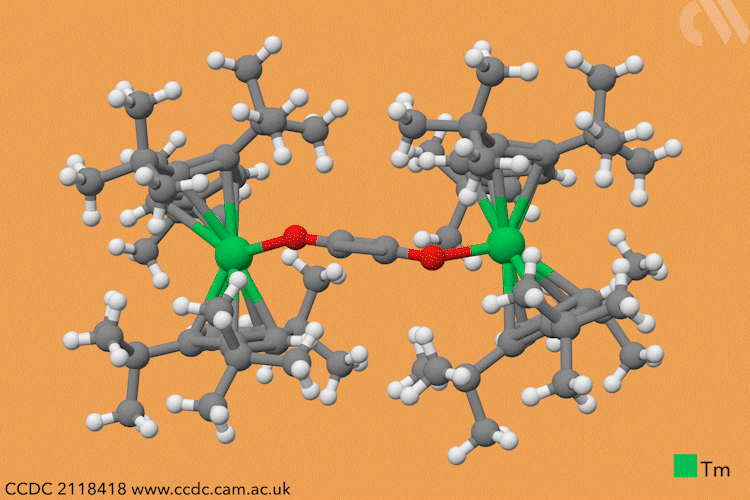
However, a team headed by Thomas Simler and Grégory Nocton from the CNRS observed an immediate reaction between their dark purple thulium complex and carbon monoxide. Analysis of the resultant brown solid revealed that two different carbon monoxide oligomer complexes had formed: one dimer and one trimer.
Simler and Nocton were keen to probe the reactivity of these polymeric products and exposed the complexes to a variety of electrophiles, producing a series of homologated systems up to six carbons in length. The most unusual reaction occurred when the team bubbled carbon dioxide through a solution of the dimer in toluene. Red crystals immediately formed, and the team isolated an unexpected tetranuclear product – a dimer of the dimer – in which C–H activation of the aromatic solvent had also occurred.
The group is now working to elucidate the mechanism of this unprecedented reactivity and hope to develop this complex for future catalytic applications.

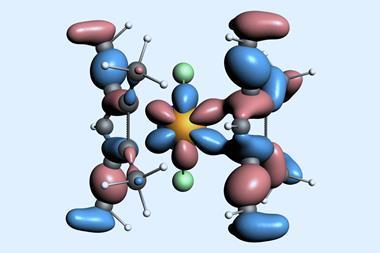
![An image showing a [M2(BzN6-Mes)]n− complex](https://d2cbg94ubxgsnp.cloudfront.net/Pictures/380x253/4/0/8/514408_index_128835.jpg)








No comments yet