Catalyst that never needs to touch its reactant breaks textbook principle
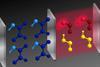
Calculations show how catalyst confined in optical cavity can influence reagent without the two ever coming into contact
‘We want to challenge the textbook paradigm that reactants and catalyst need to bind to each other to induce some chemical reactivity,’ says Joel Yuen-Zhou from the University of California, San Diego, US. Yuen-Zhou’s team has proven, at least in theory, that a catalyst trapped between two mirrors can control a reagent stuck between two adjacent mirrors – without the two ever touching. The concept could allow chemists to break bonds that they can’t access through other means.
