2022 was Italy’s driest year on record. But when a team from the University of Cambridge in the UK travelled there that July to enter their artificial tree in a competition, they were thwarted by a storm dumping a quarter of the average annual rainfall in a single day. ‘We all went to Italy together, we all looked after the device, we all saw the storm coming and we all saw our device dissolving,’ remembers Erwin Reisner, who co-led the team with Samuel Stranks.
When Reisner and his colleagues first heard about the European Innovation Council’s (EIC) Horizon Prize competition challenging people to build a functional prototype of an artificial photosynthesis-based system that produced a useable synthetic fuel, they were excited to get involved. The chance to scoop €5 million (£4.17 million) in prize money was a bonus.
A project they concluded in 2019 had seen Reisner and his co-workers develop an artificial leaf – a photoelectrochemical device that combined light harvesting and catalysis to generate syngas from sunlight, carbon dioxide and water. Realising they were on the front foot, Reisner assembled a team to develop this laboratory prototype for the EIC competition.
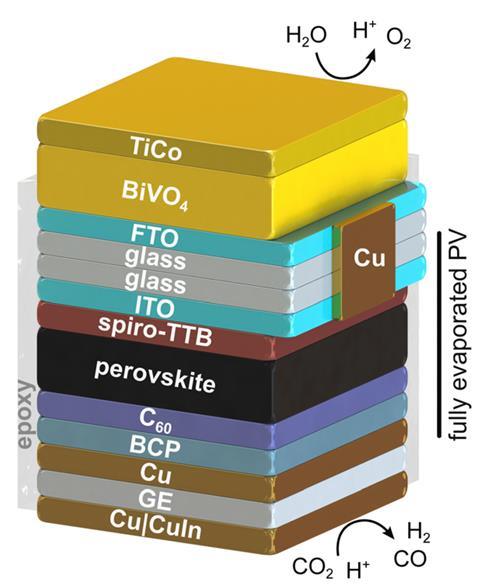
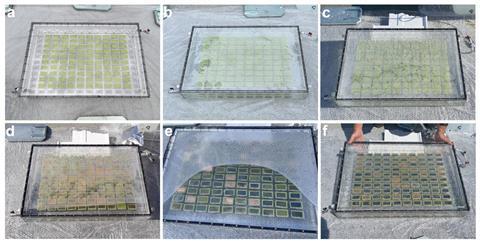
Scaling up from one leaf to an array of over 100 saw five postgraduate students and postdoctoral researchers work tirelessly for two months. The team chose to manufacture the leaves using vacuum processing due to the technique’s scalability, however, it was still a challenge. ‘I think for me this puts the emphasis now much more on scalable processing methods, rather than solely on materials … if you cannot make these square metres quickly and cheaply it will be very difficult to make things at the required scale,’ says Reisner.
The resulting artificial tree covered an area of 0.35m2 and had one hundred 0.001m2 artificial leaves. During operation, the system is filled with a potassium bicarbonate buffer solution saturated with carbon dioxide. Each leaf module has a tandem configuration consisting of a bismuth vanadate photoanode paired with a cobalt-based catalyst for water oxidation, and a perovskite photocathode paired with a copper–indium catalyst to reduce carbon dioxide.
When the sun shines on it, the system converts carbon dioxide and water into syngas, a mixture of carbon monoxide and hydrogen. It was designed to only collect pure syngas and achieves this by connecting it to two tanks via a series of valves. The first tank contains a potassium hydroxide solution to absorb excess carbon dioxide. Carbon monoxide and hydrogen bubble through the liquid and collect in the tank headspace. The second tank collects excess potassium hydroxide solution displaced from the first tank by the syngas.
If we don’t build prototypes, we probably shouldn’t expect others to engage and invest in this type of technology
Lab tests with the leaves showed promise. And in an effort to mimic conditions they were expecting in Italy, a few weeks before the final they tested a 3 x 2 leaf array on a sunny day on top of the Maxwell Centre in Cambridge. While some of the potassium hydroxide solution leaked, it was nothing compared to what happened during the final.
Italy in July
The Cambridge team was one of three invited to demonstrate a device during a three-day event at the commission’s Joint Research Centre site in Ispra, Italy. The weather was mainly overcast with a thunderstorm on the first day. Insufficient syngas production and low overnight temperatures created an underpressure that drew corrosive potassium hydroxide into the Cambridge team’s device via a faulty valve. As a result, the device degraded and the basic conditions depleted the carbon dioxide, reducing the production of syngas further still. Ultimately, the 10 x 10 array produced just a fraction of the 220ml of carbon monoxide and 910ml of hydrogen predicted by previous tests.

A team from Japan won the competition with an artificial photosynthesis system that generated methane gas, which they used to power an engine.
Valuable lessons
While the competition didn’t go to plan, the team saw it as a learning opportunity and encourage future work in this area, especially at large scales and under real-world conditions. ‘Outdoor testing with benchtop-size reactors is a very important step forward for the field. We need to break down the barriers between the standardised small-scale testing in the lab and outside demonstration,’ says Reisner. He hopes prototype devices at the square metre scale will become the new normal.
Jingshan Luo, an expert in solar fuels at Nankai University in China who wasn’t involved in the project, shares this sentiment and says the work ‘provides valuable lessons on the discrepancies between ideal laboratory settings and real-world challenges. It will likely encourage further research efforts aimed at overcoming these barriers and improving device robustness.’
‘We are used to discussing ideas and concepts based on PowerPoint slides, but it really requires a dedicated effort now to move ahead and get stuff done,’ adds Reisner. ‘If we don’t set a good example and build prototypes, we probably shouldn’t expect others to engage and invest in this type of technology.’
References
This article is open access
V Andrei et al, Energy Environ. Sci., 2025, DOI: 10.1039/d4ee05780e















No comments yet