Chemists reconsider C–H and C–C bond length rationale
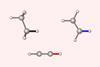
Quantitative proof for steric repulsion theory
Pauli repulsion, rather than orbital interactions, is the driving force behind why C–C and C–H bonds contract as the number of substituents surrounding the carbon centre decreases, new research shows.