Chiral benzyne made with single handedness for the first time
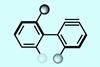
First enantioenriched aryne atropisomer can create chiral nanographene and anthracene structures
The first axially chiral benzyne – a highly reactive aromatic ring with an additional bond – has been made by chemists in France. The team proved that the atropisomeric aryne retains its stereochemical information in Diels–Alder cycloaddition reactions and can be used to create chiral polycyclic aromatic hydrocarbons including small nanographene molecules.
