New synthesis technique allows the length and stereochemistry of artificial polymers to be controlled
Creating polymers with controlled length and stereochemistry is an area where nature is currently streets ahead of science. But researchers at Massachusetts Institute of Technology (MIT) in the US have come closer to closing the gap, with an efficient strategy that allows them to synthesise a new family of unimolecular, sequence- and stereo-defined polymers using click chemistry.
Similar polymers have been created by adding one monomer at a time to a growing chain attached to a solid or soluble polymer support, but limited yields and inefficient use of reagents can result.
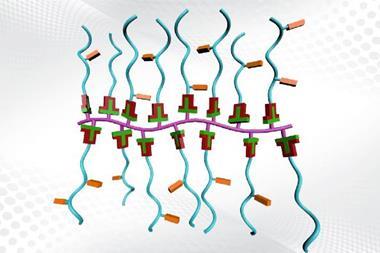
‘We utilize an iterative exponential growth strategy where instead of adding one monomer at a time we double the length of the polymer with each iteration,’ explains senior author Jeremiah A Johnson. ‘It leads to longer chains of repeating sequences in fewer steps than would be possible with other methods, and also enables the use of catalytic reactions that are more atom economical.’
The strategy has produced macromolecules with exact chain lengths before. Johnson’s new system – exponential growth plus side-chain functionalisation, or IEG+ – is exceptional in that one of the deprotection steps also includes the addition of a variable side chain. The diversity in sequence is therefore introduced during synthesis.

Side chains are the key. ‘The main chain in all biomaterials, say proteins, is always the same, but the side chains and the sequence of the side chains is what make the difference,’ says Jean-Francois Lutz, a polymer chemist at the French National Centre for Scientific Research in Paris, France, who hails this as another step toward manmade materials as complex as biomaterials.
He describes the molecular weight and refinement achievement as ‘quite high’, though nature achieves far more. ‘I expect we can fill the gap between synthetic polymer and biopolymers in a decade or two, and…create very refined polymers with controllable properties,’ says Lutz.
Understanding how sequence impact properties in specific systems will allow chemists to tune polymeric materials for applications in areas like bioengineering, molecular electronics, and catalysis.
University of Pittsburgh chemist Tara Meyer praises this IEG+ approach for producing unimolecular products nearly unobtainable outside a biological system, especially at the speed and scale reported. ‘The strengths of the Johnson approach include simplicity, molecular weight control, low number of reaction steps for a particular chain length, scalability, and modularity,’ she says. ‘The trade-offs are the fixed backbone structure, the moderate molecular weights that can be practically obtained, and the unavoidable loss of step efficiency as sequence complexity is increased.’









No comments yet