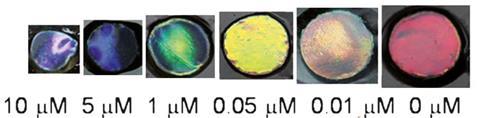
Heavy metal ions such as mercury and lead are persistent pollutants in the environment and can cause severe health problems. Routine sampling of water sources is vital to evaluate the levels of toxic metals.
Current detection techniques involve the use of expensive equipment, require skilled technicians and are time consuming. A range of research has looked at developing detection techniques for in-field use and recently, interest has been focused on using aptamers. Aptamers are single-stranded DNA or RNA molecules that have been shown to reversibly bind with mercury and lead. Several sensors have been developed that incorporate aptamers but most have required complex labelling or detection techniques.
Zhongze Gu at Southeast University, and colleagues, produced a simple sensor by combining aptamers with a colloidal photonic crystal hydrogel (CPCH) film. The sensor was red but contact with mercury or lead turned it blue. Upon binding with a metal ion, the aptamer conformation was altered. This triggered the hydrogel to shrink, which in turn caused the colloidal crystals to change colour.
‘The specific binding of heavy metal ions and the cross-linked single-stranded aptamers in the CPCHs cause hydrogel shrinkage, which induces a decrease in the lattice spacing of the CPCHs. This is observed as a blue shift of the structural colours,’ Gabriela Telipan at the National Research and Development Institute for Electrical Engineering, Romania, explains further. Telipan is an expert in sensor materials.
The sensor is simple to use and requires no specialist equipment as the colour change is detectable with the naked eye. Gu adds that in the future, they will adapt the sensor for other analyses such as drugs, food additives and pesticides. ‘We hope we can achieve multiplexed visual detection for different analytes simultaneously,’ he says.






No comments yet