A computational study has replaced the carbon in cytosine, guanine, adenine, thymine and deoxyribose with nitrogen and boron to understand what carbonless DNA might be like. The study generated a set of structures that were deemed geometrically and electrostatically comparable to conventional DNA molecules.
Piotr Skurski and Jakub Brzeski from the University of Gdańsk in Poland assessed the structural suitability and molecular interactions of the carbonless structures using density functional theory (DFT) calculations, then validated them using more accurate methods.
Complementary base pairing of the carbonless nucleotide analogues and a carbonless DNA dimer proved to be geometrically comparable to conventional DNA molecules, with minor changes to the molecular electrostatic potential distribution in the carbonless DNA dimer.
A carbonless DNA hexamer strongly mimicked its conventional DNA equivalent except its twist angle was slightly larger at 39° compared with 35°. Furthermore, docking simulations suggest that carbonless DNA’s binding affinity may be greater for polar molecules due to a shift in polarisation caused by the B–N substitution.
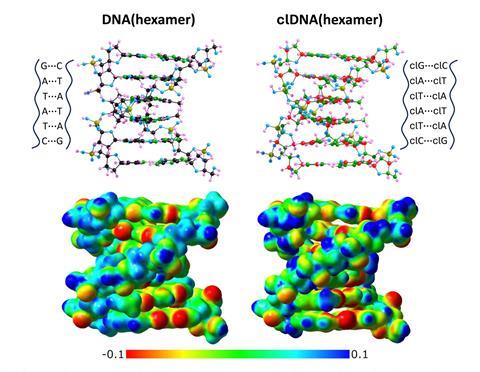
Developing such carbonless analogues could spark the creation of alternative biological frameworks for gene therapeutics and drug delivery. Research into carbonless genetic information storage may also inform on the possibilities of life on other planets. And, as Skurski and Brzeski suggest, this work challenges assumptions that carbon has to be the central building block of life.
References
P Skurski and J Brzeski, Phys. Chem. Chem. Phys., 2025, DOI: 10.1039/d4cp04410j












No comments yet