Crystals allow peek at picosecond DNA damage
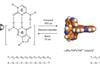
Combined x-ray and laser technique explores reaction with light lasting trillionths of a second

Combined x-ray and laser technique explores reaction with light lasting trillionths of a second