Dimetallocene with two different metal centres synthesised for the first time
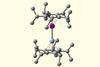
Molecule first theorised 20 years ago created in the lab
Even though thousands of metallocenes – sandwich molecules with a central metal atom – exist, only now has a sandwich structure containing two different metal atoms been synthesised. This new research provides a deeper understanding of the underlying chemistry of these complex bonds and offers a blueprint to stabilise other similar theorised compounds.
Metallocenes comprise two cyclopentandienyl anions (Cp-) bound to a metal centre. Ferrocene is the archetypal metallocene where each Cp ligand forms five metal bonds with the iron centre via overlapping π orbitals. Other transition metal ions also form metallocenes similar to ferrocene including nickel, ruthenium and osmium. The stability of these structures varies with the metal and its oxidation state, with the greatest stability achieved when the electronic configuration resembles a noble gas.
