Interrupting a fungus-produced ‘nitric oxide (NO) burst’ may be key to saving the world’s most popular banana from extinction. Research into how a fungus both causes Panama disease and protects itself from nitric oxide-induced stress could open new avenues to combat the destructive infection in banana plants.
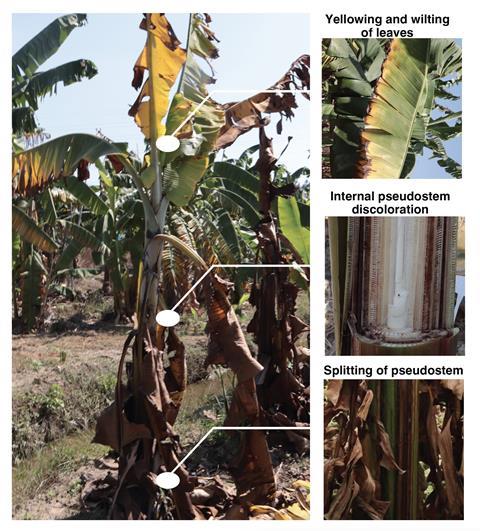
To produce fruits with minimal seeds, bananas are propagated by cloning, resulting in very little genetic diversity. Combined with the dense monoculture plantations they’re primarily grown in this leaves bananas extremely vulnerable to diseases and pests. One major threat is Fusarium wilt, also known as Panama disease, caused by Fusarium oxysporum (Foc). One strain of Foc – race 1 (R1) – was responsible for the devastation of Gros Michel bananas in the 1950s. The Cavendish variety of bananas was selected as the Gros Michel replacement based on its R1 resistance and has become the most popular cultivated banana worldwide. However, in 1989 another strain of Foc – tropical race 4 (TR4) – capable of infecting Cavendish bananas was reported. TR4 has spread from its suspected origin in Indonesia and Malaysia across Asia to the Middle East, Africa and Oceania. In 2019 it was detected in Columbia, and in 2021 in Peru, the two largest Cavendish banana exporting regions in the world. The dominant banana variety once again faces functional extinction due to Panama disease.
Hoping to gain insight into how TR4 is able to infest Cavendish banana plants, researchers compared the genomic information for 36 Foc strains collected from locations around the world. While most Foc strains increase their virulence through horizontally acquired accessory chromosomes, the researchers found that TR4 had none. Instead, its genome features accessory genes attached to the ends of several core chromosomes. These genes are rich in sequences with known mitochondrial functions, particularly those related to nitric oxide biosynthesis and encoding proteins protective against nitrosative stress.
The researchers hypothesise that nitric oxide plays a key role in the TR4-Cavendish interaction, with the banana plant first recognising signals of fungal invasion and activating defence mechanisms that involve a jasmonic acid signalling pathway. However, the plant-produced methyl jasmonate (pictured below) stimulates fungal NO production in TR4 thanks to its acquired accessory genes. The resulting ‘NO burst’, causes nitrosative stress within banana roots, but not within TR4 due to the upregulation of its detoxification genes.
To test their theory, the team measured NO production in TR4 and R1 after exposure to methyl jasmonate. Only TR4 showed a burst of NO production. Similarly, knocking out genes involved in the NO biosynthesis pathway in TR4 created mutants with significantly reduced virulence.
The team believes that NO scavengers may be one potential treatment option for slowing or stopping the TR4 invasion of Cavendish bananas.
References
Y Zhang et al, Nat. Microbiol., 2024, DOI: 10.1038/s41564-024-01779-7















No comments yet