Double heptagon-containing fullerenes produced by low pressure combustion
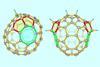
Study finds identical nonclassical fullerene cores can created by separate processes
Scientists in China have isolated a double heptagon-containing C70H6 fullerene from the soot of a benzene and acetylene mixture that has combusted in low-pressure conditions.