Dysprosium molecular magnet sets new temperature record
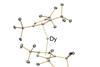
Sandwich compound keeps its magnetic memory at –213°C, bringing ultra-dense data storage a step closer
A sandwich-like dysprosium compound is the hottest single molecule magnet ever made. It stays magnetised up to –213°C – still a trifle chilly when compared with room temperature, but more than four times warmer than the previous record holder. Single molecule magnets like this could revolutionise data storage.
