Electric shock brings diamines to life
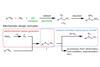
A combination of electrochemistry and organic catalysis transforms alkenes into hard-to-make vicinal diamines
Organic chemists in the US have zapped alkenes with an electrical current to make vicinal diamines. These useful structures appear in many bioactive molecules, such as the antiarrhythmic lidocaine.
